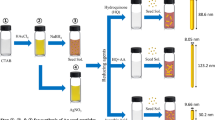
Abstract
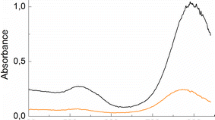
The formation of gold nanorods (AuNRs) has recently attracted great attention due to their shape-dependent optical properties that are important for many applications. The development of simpler and safer methods for the high-yield synthesis of AuNRs employing low-cost and easily handled reagents is thus of great importance. Here, we introduce, for the first time, a one-pot seedless method for the preparation of single-crystalline AuNRs in almost 100 % yield based on the use of glycerol in alkaline medium as an eco-friendly, low-cost and pH-tunable reducing agent. The synthesized AuNRs were characterized by UV–Vis–NIR spectroscopy, FEG–SEM and HRTEM. The effect of the presence of capping agent (CTAB) and the concentration of reactants (glycerol, NaOH and AgNO3) on the yield and aspect ratio (AR) of AuNRs is discussed. The AR and yield of AuNRs showed a clear dependence on the pH and temperature of the reaction mixture as well as on the concentration of AgNO3 added as an auxiliary reagent. The longitudinal plasmon resonance band of the resulting AuNRs can be tuned between 620 and 1200 nm by varying the reaction conditions. AuNRs with an aspect ratio (AR) of around 4 were obtained in almost 100 % yield at room temperature and under mild reducing environment. The formation of AuNRs is faster at higher pH (>11) and higher temperature (>30 °C), but the AuNR yield is smaller (<70 %). Variation in the pH of the reaction mixture in the range 12–13.5 results in the formation of AuNRs with different ARs and in different yields (27–99 %). Detailed study of the AuNRs crystallography by HRTEM showed that the AuNRs grow in [001] direction and have a perfect single-crystalline fcc structure, free from structural faults or dislocations. The present green method, which introduces glycerol as a tunable reducing agent with a pH-dependent reducing power, can provide a more general strategy for the preparation of a wide range of metallic nanoparticles.
Graphical abstract










Similar content being viewed by others
References
Abdelrasoul GN, Cingolani R, Diaspro A et al (2014) Photochemical synthesis: effect of UV irradiation on gold nanorods morphology. J Photochem Photobiol A Chem 275:7–11. doi:10.1016/j.jphotochem.2013.10.008
Albanese A, Tang PS, Chan WCW (2012) The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng 14:1–16. doi:10.1146/annurev-bioeng-071811-150124
Al-thabaiti SA, Hussain JI, Hashmi AA, Khan Z (2013) Au (III)-surfactant complex-assisted anisotropic growth of advanced platonic Au-nanoparticles. Can Chem Trans 1:238–252
Andrievskii RA, Khachoyan AV (2010) Role of size-dependent effects and interfaces in physicochemical properties of consolidated nanomaterials. Russ J Gen Chem 80:555–566. doi:10.1134/S1070363210030370
Blatchford CG, Campbell JR, Creighton JA (1982) Plasma resonance—enhanced raman scattering by absorbates on gold colloids: the effects of aggregation. Surf Sci 120:435–455. doi:10.1016/0039-6028(82)90161-3
Burda C, Chen X, Narayanan R, El-Sayed MA (2005) Chemistry and properties of nanocrystals of different shapes. Chem Rev 105:1025–1102. doi:10.1021/cr030063a
Busbee BD, Obare SO, Murphy CJ (2003) An improved synthesis of high-aspect-ratio gold nanorods. Adv Mater 15:414–416. doi:10.1002/adma.200390095
Cao J, Sun T, Grattan KTV (2014) Gold nanorod-based localized surface plasmon resonance biosensors: a review. Sens Actuators B Chem 195:332–351. doi:10.1016/j.snb.2014.01.056
Carbó-Argibay E, Rodríguez-González B, Gómez‐Graña S et al (2010) The crystalline structure of gold nanorods revisited: evidence for higher-index lateral facets. Angew Chem Int Ed 49:9397–9400. doi:10.1002/anie.201004910
Chang S-S, Lee C-L, Wang CRC (1997) Gold nanorods: electrochemical synthesis and optical properties. J Phys Chem B 101:6661–6664. doi:10.1021/jp971656q
Chang S-S, Shih C-W, Chen C-D et al (1999) The shape transition of gold nanorods. Langmuir 15:701–709. doi:10.1021/la980929l
Choi J, Yang J, Jang E et al (2011) Gold nanostructures as photothermal therapy agent for cancer. Anticancer Agents Med Chem 11:953–964
Creighton JA, Eadont DG, Eadon DG (1991) Ultraviolet-visible absorption spectra of the colloidal metallic elements. J Chem Soc, Faraday Trans 87:3881–3891. doi:10.1039/ft9918703881
Cuenya BR (2010) Synthesis and catalytic properties of metal nanoparticles: size, shape, support, composition, and oxidation state effects. Thin Solid Films 518:3127–3150. doi:10.1016/j.tsf.2010.01.018
Daniel M-C, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104:293–346. doi:10.1021/cr030698+
Das I, Ansari SA (2009) Nanomaterials in science and technology. J Sci Ind Res 68:657–667
El-Brolossy TA, Abdallah T, Mohamed MB et al (2008) Shape and size dependence of the surface plasmon resonance of gold nanoparticles studied by Photoacoustic technique. Eur Phys J Spec Top 153:361–364. doi:10.1140/epjst/e2008-00462-0
Foss CA, Hornyak GL, Stockert JA, Martin CR (1992) Optical properties of composite membranes containing arrays of nanoscopic gold cylinders. J Phys Chem 96:7497–7499. doi:10.1021/j100198a004
Garcia AC, Gasparotto LHS, Gomes JF, Tremiliosi-Filho G (2012) Straightforward synthesis of carbon-supported Ag nanoparticles and their application for the oxygen reduction reaction. Electrocatalysis 3:147–152. doi:10.1007/s12678-012-0096-z
Garcia AG, Lopes PP, Gomes JF et al (2014) Eco-friendly synthesis of bimetallic AuAg nanoparticles. New J Chem 38:2865–2873. doi:10.1039/c4nj00041b
Garg N, Scholl C, Mohanty A, Jin R (2010) The role of bromide ions in seeding growth of Au nanorods. Langmuir 26:10271–10276. doi:10.1021/la100446q
Gasparotto LHS, Garcia AC, Gomes JF, Tremiliosi-Filho G (2012) Electrocatalytic performance of environmentally friendly synthesized gold nanoparticles towards the borohydride electro-oxidation reaction. J Power Sources 218:73–78. doi:10.1016/j.jpowsour.2012.06.064
Gole A, Murphy CJ (2004) Seed-mediated synthesis of gold nanorods: role of the size and nature of the seed. Chem Mater 16:3633–3640. doi:10.1021/cm0492336
Gomes JF, Garcia AC, Ferreira EDB et al (2015) New insight into the formation mechanism of the Ag, Au and AgAu nanoparticles in aqueous alkaline media: alkoxides from alcohols, aldehydes and ketones as the universal reducing agent. Phys Chem Chem Phys. doi:10.1039/C5CP02155C
Hebbalalu D, Lalley J, Nadagouda MN, Varma RS (2013) Greener techniques for the synthesis of silver nanoparticles using plant extracts, enzymes, bacteria, biodegradable polymers, and microwaves. ACS Sustain Chem Eng. doi:10.1021/sc4000362
Huang X, El-Sayed IH, El-Sayed MA (2010) Applications of gold nanorods for cancer imaging and photothermal therapy. Methods Mol Biol 624:343–357. doi:10.1007/978-1-60761-609-2_23
Hvolbæk B, Janssens T, Clausen B (2007) Catalytic activity of Au nanoparticles. Nano Today 2:14–18
Jana NR, Gearheart L, Murphy CJ (2001a) Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Adv Mater 13:1389–1393. doi:10.1002/1521-4095(200109)13:18<1389:AID-ADMA1389>3.0.CO;2-F
Jana NR, Gearheart L, Murphy CJ (2001b) Wet-chemical synthesis of high aspect ratio cylindrical gold nanorods. J Phys Chem B 105:4065–4067
Johnson CJ, Dujardin E, Davis SA et al (2002) Growth and form of gold nanorods prepared by seed-mediated, surfactant-directed synthesis. J Mater Chem 12:1765–1770. doi:10.1039/b200953f
Kah JCY, Yeo ELL, He S, Engudar G (2015) Gold nanorods in photomedicine. Appl Nanosci Photomed. doi:10.1533/9781908818782.221
Kalyan Kamal SS, Vimala J, Sahoo PK et al (2014) A green chemical approach for synthesis of shape anisotropic gold nanoparticles. Int Nano Lett 4:109. doi:10.1007/s40089-014-0109-4
Kang SK, Chah S, Yun CY, Yi J (2003) Aspect ratio controlled synthesis of gold nanorods. Korean J Chem Eng 20:1145–1148. doi:10.1007/BF02706952
Kannadorai RK, Chiew GGY, Luo KQ, Liu Q (2015) Dual functions of gold nanorods as photothermal agent and autofluorescence enhancer to track cell death during plasmonic photothermal therapy. Cancer Lett 357:152–159. doi:10.1016/j.canlet.2014.11.022
Kim F, Song JH, Yang P (2002) Photochemical synthesis of gold nanorods. J Am Chem Soc 124:14316–14317
Kim J-Y, Ah CS, Jang D-J (2011) Controlled aspect ratios of gold nanorods in reduction-limited conditions. J Nanomater 2011:1–7. doi:10.1155/2011/405853
Kotani H, Hanazaki R, Ohkubo K et al (2011) Size- and shape-dependent activity of metal nanoparticles as hydrogen-evolution catalysts: mechanistic insights into photocatalytic hydrogen evolution. Chemistry 17:2777–2785. doi:10.1002/chem.201002399
Kou J, Verma RS (2013) Speedy fabrication of diameter-controlled Ag nanowires using glycerol under microwave irradiation conditions. Chem Commun 49:692–694. doi:10.1039/c2cc37696b
Kou J, Bennett-Stamper C, Varma RS (2013) Green synthesis of noble nanometals (Au, Pt, Pd) using glycerol under microwave irradiation conditions. ACS Sustain Chem Eng 816:400007. doi:10.1021/sc400007p
Link S, Mohamed MB, El-Sayed MA (1999) Simulation of the optical absorption spectra of gold nanorods as a function of their aspect ratio and the effect of the medium dielectric constant. J Phys Chem B 103:3073–3077. doi:10.1021/jp990183f
Liu M, Guyot-Sionnest P (2005) Mechanism of silver(I)-assisted growth of gold nanorods and bipyramids. J Phys Chem B 109:22192–22200. doi:10.1021/jp054808n
Marks L (1994) Experimental studies of small particle structures. Reports Prog Phys 57:603–649
Mohamed MB, Volkov V, Link S, El-Sayed MA (2000) The ‘lightning’ gold nanorods: fluorescence enhancement of over a million compared to the gold metal. Chem Phys Lett 317:517–523. doi:10.1016/S0009-2614(99)01414-1
Moulton MC, Braydich-Stolle LK, Nadagouda MN et al (2010) Synthesized silver nanoparticles using tea polyphenols. Green Chem 2:763–770
Murphy CJ, Sau TK, Gole AM et al (2005) Anisotropic metal nanoparticles: synthesis, assembly, and optical applications. J Phys Chem B 109:13857–13870. doi:10.1021/jp0516846
Murphy CJ, Gole AM, Stone JW et al (2008) Gold nanoparticles in biology: beyond toxicity to cellular imaging. Acc Chem Res 41:1721–1730. doi:10.1021/ar800035u
Ng KC, Cheng W (2012) Fine-tuning longitudinal plasmon resonances of nanorods by thermal reshaping in aqueous media. Nanotechnology 23:105602. doi:10.1088/0957-4484/23/10/105602
Nikoobakht B, El-Sayed M (2003) Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem Mater 15:1957–1962
Orendorff CJ, Gearheart L, Jana NR, Murphy CJ (2006) Aspect ratio dependence on surface enhanced Raman scattering using silver and gold nanorod substrates. Phys Chem Chem Phys 8:165–170. doi:10.1039/b512573a
Perez-Juste J, Pastoriza-Santos I, Liz-Marzan LM, Mulvaney P (2005) Gold nanorods: synthesis, characterization and applications. Coord Chem Rev 249:1870–1901
Salamakha LP, Bauer E, Mudryi SI et al (2009) Isothermal section of the Ce-Au-Sb system at 870 K. J Alloys Compd 479:184–188
Samim MM, Prashant CK, Dinda AK et al (2011) Synthesis and characterization of gold nanorods and their application for photothermal cell damage. Int J Nanomed 6:1825–1831. doi:10.2147/IJN.S11600
Sánchez‐Iglesias A, Grzelczak M, Pérez‐Juste J, Liz‐Marzán LM (2010) Binary self-assembly of gold nanowires with nanospheres and nanorods. Angew Chem Int Ed 49:9985–9989. doi:10.1002/anie.201005891
Sau TK, Murphy CJ (2004) Seeded high yield synthesis of short Au nanorods in aqueous solution. Langmuir 20:6414–6420. doi:10.1021/la049463z
Shekhar H (2013) Thermodynamics of gold nanoparticle growth: a first-principles investigation. Chalmer University of Technology, Goteborg
Wang Z, Mohamed M, Link S, El-Sayed M (1999) Crystallographic facets and shapes of gold nanorods of different aspect ratios. Surf Sci 440:L809–L814. doi:10.1016/S0039-6028(99)00865-1
Wang ZL, Gao RP, Nikoobakht B, El-Sayed MA (2000) Surface reconstruction of the unstable 110 surface in gold nanorods. J Phys Chem B 104:5417–5420. doi:10.1021/jp000800w
Xiao J, Qi L (2011) Surfactant-assisted, shape-controlled synthesis of gold nanocrystals. Nanoscale 3:1383–1396. doi:10.1039/c0nr00814a
Zhang Y, Yu J, Birch DJS, Chen Y (2010) Gold nanorods for fluorescence lifetime imaging in biology. J Biomed Opt 15:0205041–0205043. doi:10.1117/1.3366646
Zhang L, Xia K, Lu Z et al (2014) Efficient and facile synthesis of gold nanorods with finely tunable plasmonic peaks from visible to near-IR range. Chem Mater 26:1794–1798. doi:10.1021/cm403109k
Acknowledgments
R. Parveen and S. Ullah are thankful to The World Academy of Science (TWAS, Italy) and National Council for Scientific and Technological Development (CNPq, Brazil) for PhD fellowship. J.F. Gomes thanks FAPESP for the postdoctoral fellowship (Process Number: 2009/08511-9). The authors also thank Professor Andre Avelino Pasa and Cristiani Campos Plá Cid at UFSC, SC, Brazil, for HRTEM analysis and Teko Wilhelmin Napporn for his guidance and assistance in the initial stages of this work.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Parveen, R., Gomes, J.F., Ullah, S. et al. One-pot high-yield synthesis of single-crystalline gold nanorods using glycerol as a low-cost and eco-friendly reducing agent. J Nanopart Res 17, 418 (2015). https://doi.org/10.1007/s11051-015-3223-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-3223-y