Abstract
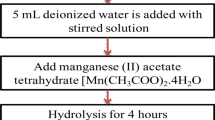
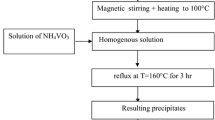
A new approach has been developed to synthesize manganese-containing titanium dioxide materials by hydrolysis of titanyl sulfate. The samples were studied by a complex of methods (synchrotron radiation X-ray powder diffraction, high-resolution scanning electron microscopy with energy-dispersive X-ray spectroscopy, absorption spectroscopy). The sequence of the added reagents effects the phase composition (anatase or mixtures of anatase and “η-TiO2”), size of crystallites, nanoparticles and agglomerates, manganese content, and oxidation state (Mn3+, Mn2+/Mn3+, or Mn3+/Mn4+). The Mn-doped TiO2 samples have been proven to have high photocatalytic activity for methyl orange (MO) under visible light. The rate of MO degradation reached 0.0046 min−1 (50 % in 150 min) for the sample containing a mixture of anatase (75 %) and “η-TiO2” (25 %) with a high degree of amorphism; the sample is characterized by the smallest size of crystallites (44.3 Å), the largest size of nanoparticles (33 nm) and agglomerates (10 μm), and the lowest manganese content (0.3 at. %) with the ratio Mn3+:Mn4+ = 1:1. The resultant Mn-doped titania has potential applications in photocatalysis and environmental protection.
Graphical Abstract








Similar content being viewed by others
References
Baranchikov AE, Ivanov VK, Tret’yakov YD (2012) Hydrothermal microwave synthesis of nanocrystalline anatase. Dokl Chem 447:241–243
Brus VV, Kovalyuk ZD, Maryanchuk PD (2012) Optical properties of TiO2–MnO2 thin films prepared by electron-beam evaporation. Tech Phys 57:1148–1151
Cargnello M, Gordon TR, Murray CB (2014) Solution-phase synthesis of titanium dioxide nanoparticles and nanocrystals. Chem Rev 114:9319–9345
Chernyshov AA, Veligzhanin AA, Zubavichus YV (2009) “Structural materials science” end-station at the Kurchatov synchrotron radiation source: recent instrumentation upgrades and experimental results. Nucl Instr Meth Phys Res A 603:95–98
Dadachov M (2006) United States Patent Application Publication. US 2006/0171877
Dambournet D, Belharouak I, Amine K (2010) Tailored preparation methods of TiO2 anatase, rutile, brookite: mechanism of formation and electrochemical properties. Chem Mater 22:1173–1179
Deng QR, Xia XH, Guo ML, Gao Y, Shao G (2011) Mn-doped TiO2 nanopowders with remarkable visible light photocatalytic activity. Mater Lett 65:2051–2054
Dzis’ko VA, Karnaukhov AP, Tarasova DV (eds) (1978) Physicochemical fundamentals for the synthesis of oxide catalysts. Nauka, Novosibirsk
Ekoko GB, Lobo JK-K, Mvele OM, Muswema JL, Yamambe J-FS (2014) Effect of an external applied potential on the photocatalytic properties of manganese-doped titanium dioxide. Am J Phys Chem 3:41–46
Eliseev AA, Lukashin AV (2010) Functional nanomaterials. Fizmatlit, Moscow
Gusev AI (2009) Nanomaterials, nanostructures, nanotechnology. Fizmatlit, Moscow
Hammersley AP, Svensson SO, Hanfland M, Fitch AN, Hausermann D (1996) Two-dimensional detector software: from real detector to idealized image of two-theta scan. High Press Res 14:235–248
Hao L, Wei H (2010) On-line investigation of anatase precipitation from titanyl sulphate solution. Chem Eng Res Des 88:1264–1271
Ismagilov ZR, Tsikoza LT, Shikina NV, Zarytova VF, Zinoviev VV, Zagrebelnyi SN (2009) Synthesis and stabilization of nano-sized titanium dioxide. Rus Chem Rev 78:873–885
Ivanov VK, Maksimov VD, Shaporev AS, Baranchikov AE, Churagulov BP, Zvereva IA, Tret’yakov YD (2010) Hydrothermal synthesis of efficient TiO2-based photocatalysts. Russ J Inorg Chem 55:150–154
Jin Q, Arimoto H, Fujishima M, Tada H (2013) Manganese oxide-surface modified titanium(IV) dioxide as environmental catalyst. Catalysts 3:444–454
Kho YK, Iwase A, Teoh WY, Madler L, Kudo A, Amal R (2010) Photocatalytic H2 evolution over TiO2 nanoparticles. The synergistic effect of anatase and rutile. J Phys Chem C 114:2821–2829
Kijlstra WS, Poels EK, Bliek A, Weckhuysen BM, Schoonheydt RA (1997) Characterisation of Al2O3-supported manganese oxides by electron spin resonance and diffuse reflectance spectroscopy. J Phys Chem B 101:309–316
Kuz’micheva GM, Savinkina EV, Obolenskaya LN, Belogorokhova LI, Mavrin BN, Chernobrovkin MG, Belogorokhov AI (2010) Preparation, characterization and properties of nano-sized titanium dioxide modifications with the structures of anatase and η-TiO2. Crystallogr Rep 55:866–871
Kuz’micheva GM, Savinkina EV, Belogorokhova LI, Mavrin BN, Flid VR, Yakovenko AG, Belogorokhov AI (2011) The characteristics of the nanosized eta-TiO2 polymorph. Russ J Phys Chem A 85:1037–1040
Kuz’micheva GM, Gainanova AA, Orekhov AS, Klechkovskaya VV, Sadovskaya NV, Chernyshev VV (2014) Peculiarities of the microstructure of a nanoscale modification of η-TiO2. Cryst Rep 59:916–922
Lee C, Aikens CM (2013) Effects of Mn doping on (TiO2) n (n = 2–5) complexes. Comp Theor Chem 1013:32–45
Li HM, Liu M, Zeng YS, Huang TC (2010) Coexistence of antiferromagnetic and ferromagnetic in Mn-doped anatase TiO2 nanowires. J Cent South Univ Technol 17:239–243
Luca V, Djajanti S, Howe RF (1998) Structural and electronic properties of sol–gel titanium oxides studied by X-ray absorption spectroscopy. J Phys Chem B 102:10650–10657
Luca V (2009) Comparison of size-dependent structural and electronic properties of anatase and rutile nanoparticles. J Phys Chem C 113:6367–6380
Maksimov VD, Shaporev AS, Ivanov VK, Churagulov BR, Tret’yakov YD (2009) Hydrothermal synthesis of nanocrystalline anatase from aqueous solutions of titanyl sulfate for photocatalytic applications. Theor Found Chem Eng 43:713–718
Milella F, Gallardo-Amores JM, Baldic M, Busca G (1998) A study of Mn–Ti oxide powders and their behaviour in propane oxidation catalysis. J Mater Chem 8:2525–2531
Ngamta S, Boonprakob N, Wetchakun N, Ounnunkad K, Phanichphant S, Inceesungvorn B (2013) A facile synthesis of nanocrystalline anatase TiO2 from TiOSO4 aqueous solution. Mater Lett 105:76–79
Nie X, Zhuo Sh, Maeng G, Sohlberg K (2009) Doping of TiO2 polymorphs for altered optical and photocatalytic properties. Int J Photoenergy. doi:10.1155/2009/294042
Obolenskaya LN, Kuz’micheva GM, Savinkina EV, Prokudina NA, Chernyshov VV, Sadovskaya NV (2012a) Effect of sulfate synthesis conditions on characteristics of samples with the nanosized eta-TiO2 modification. Russ J Inorg Chem 57:1177–1181
Obolenskaya LN, Kuz’micheva GM, Savinkina EV, Sadovskaya NV, Zhilkina AV, Prokudina NA, Chernyshev VV (2012b) Influence of the conditions of the sulfate method on the characteristics of nanosized anatase-type samples. Russ Chem Bull 61:2049–2055
Obolenskaya LN, Savinkina EV, Kuzmicheva GM (2014) Degradation of fungicides and other containments under visible light with new nano-titania photocatalysts. In: Proceedings of the 1st international symposium on nanoparticles/nanomaterials and applications, Caparica, Portugal, pp 386–387
Papadimitriou VC, Stefanopoulos VG, Romanias MN, Papagiannakopoulos P, Sambani K, Tudose V, Kiriakidis G (2011) Determination of photo-catalytic activity of un-doped and Mn-doped TiO2 anatase powders on acetaldehyde under UV and visible light. Thin Solid Films 520:1195–1201
Pu H, Huang SH, Tian CX (2014) Synthesis of titania from industrial titanyl sulfate solution and influence studies of calcination temperature. Adv Mater Res 1030–1032:193–196
Ravel B, Newville M (2005) ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J Synchrotron Rad 12:537–541
Sakthivel S, Hidalgo MC, Bahnemann DW, Geissen S-U, Murugesan V, Vogelpohl A (2006) A fine route to tune the photocatalytic activity of TiO2. Appl Catal B 63:31–40
Savinkina EV, Kuz’micheva GM, Tabachkova NY, Obolenskaya LN, Demina PA, Yakovenko AG (2011) Synthesis and morphology of anatase and η-TiO2 nanoparticles. Inorg Mater 47:489–494
Scanlon DO, Dunnill CW, Buckeridge J, Shevlin SA, Logsdail AJ, Woodley SM, Catlow CR, Powell MJ, Palgrave RG, Parkin IP, Watson GW, Keal TW, Sherwood P, Walsh A, Sokol AA (2013) Band alignment of rutile and anatase TiO2. Nat Mater 12:798–801
Shahmoradi B, Negahdary M, Maleki A (2012) Hydrothermal synthesis of surface-modified, manganese-doped TiO2 nanoparticles for photodegradation of Methylene Blue. Environ Eng Sci 29:1038–1045
Trofimova NN, Veligzhanin AA, Murzin VY, Chernyshov AA, Khramov EV, Zabluda VN, Edel’man IS, Slovokhotov YL, Zubavichus YV (2013) Structural diagnostics of functional nanomaterials with the use of X-ray synchrotron radiation. Nanotechnol Russ 8:396–401
Varma RS, Baruwati B, Virkutyte J (2011) United States Patent Application Publication. US 2011/0266136
Velu S, Shah N, Jyothi TM, Sivasanker S (1999) Effect of manganese substitution on the physicochemical properties and catalytic toluene oxidation activities of Mg–Al layered double hydroxides. Microporous Mesoporous Mater 33:61–75
Villars P (2014) Material Phases Data System (MPDS), CH-6354 Vitznau, Switzerland; Springer materials; sd_1627926. Springer, Heidelberg
Xia XH, Lu L, Walton AS, Ward M, Han XP, Brydson R, Luo JK, Shao G (2012) Origin of significant visible-light absorption properties of Mn-doped TiO2 thin films. Acta Mater 60:1974–1985
Zaleska (2008) A Doped-TiO2: a review. Recent Patents Eng 2:157–164
Zamiri G, Zakaria A, Hussein MZB, Zamiri R, Rebelo A, Ahangar H (2014) Effect of manganese doping on optical and magnetic properties of titanium dioxide nanostructures prepared by hydrothermal technique in the presence of thiourea. Micro Nano Lett 9:906–908
Zhang J, Xu Q, Feng ZC, Li MJ, Li C (2008) Importance of the relationship between surface phases and photocatalytic activity of TiO2. Angew Chem Int Ed 47:1766–1769
Acknowledgments
The study was supported by the Russian Foundation for Basic Research (project 13-03-00367).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuzmicheva, G.M., Savinkina, E.V., Obolenskaya, L.N. et al. Synthesis of Mn-sensitized TiO 2 nanoparticles: influence of sequence of reagents on phase composition and photocatalytic activity . J Nanopart Res 17, 406 (2015). https://doi.org/10.1007/s11051-015-3211-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-3211-2