Abstract
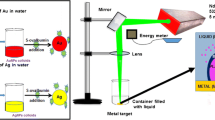
Pure gold and silver nanoparticle (NP) generation and their conjugation with protein S-ovalbumin using in situ conjugation process have been reported. The in situ conjugation involves nanosecond pulse laser ablation of pure metal target in the protein S-ovalbumin solution. Transmission electron microscopy (TEM) and UV–Visible absorption results show decrease in mean NP size along with narrow particle size distribution on ablation in S-ovalbumin solution as compared to ablation in water for both Au and Ag NPs. Also, the NP size reduction was found to be dependent on the concentration of S-ovalbumin. For AuNPs, spherical NPs of mean size 4 nm with particle size distribution 2–6 nm were obtained at 300 nM S-ovalbumin concentration. Further, it has been observed that the resultant in situ-conjugated colloid gold and silver NP solutions were quite stable even in the presence of NaCl at physiological salt concentration (0.15 M). On post-laser irradiation (532 nm, 15 mJ) for 20 min, 9 nm red shift in surface plasmon resonance peak (SPR), along with increased broadening towards longer wavelength, was observed in the AuNPs–S-ovalbumin sample. Further increase in the time of irradiation showed shift in AuNPs–S-ovalbumin SPR towards lower wavelength. On laser irradiation (532 nm, 15 mJ) for 20 min, no significant change was observed in the line shape of the plasmon absorption band of the AgNPs–S-ovalbumin conjugate. FTIR spectra revealed that S-ovalbumin peptide backbone and secondary structure remain unchanged on laser irradiation during in situ conjugation process. Thus, integrity of S-ovalbumin does not get affected, and no degradation of S-ovalbumin takes place on laser-induced in situ conjugation. Raman results confirm that both Au and Ag NPs interact with S-ovalbumin via thiol-bearing cysteine residues of the disulfide bond.













Similar content being viewed by others
References
Amendola V, Meneghetti M (2007) Controlled size manipulation of free gold nanoparticles by laser irradiation and their facile bioconjugation. J Mater Chem 17:4705–4710
Biswas N, Waring AJ, Walther FJ, Dluhy RA (2007) Structure and conformation of the disulfide bond in dimeric lung surfactant peptides SP-B1-25 and SP-B8-25. Biochim Biophys Acta 1768:1070–1082
Burt JL, Wing CG, Yoshida MM, Yacama MJ (2004) Noble-metal nanoparticles directly conjugated to globular proteins. Langmuir 20:11778–11783
Chen MC, Lord RC (1976) Laser-excited Raman spectroscopy of biomolecules. VIII. Conformational study of bovine serum albumin. J Am Chem Soc 98:990–992
Clark RJ, Hester RE (1986) Spectroscopy of biological systems. Wiley, New York
Dolgaev SI, Simakin AV, Voronov VV, Shafeev GA, Bozon- Verduraz F (2002) Nanoparticles produced by laser ablation of solids in liquid environment. Appl Surf Sci 186:546–551
Eah SK, Jaeger HM, Scherer NF, Lin XM, Wiederrecht GP (2004) Femtosecond transient absorption dynamics of close-packed gold nanocrystal monolayer arrays. Chem Phys Lett 386:390–395
Evans DF, Wennerstro H (1999) The colloidal domain: where physics, chemistry, biology and technology meet, 2nd edn. Wiley, New York
Fothergill LA, Fothergill JE (1970) Thiol and disulfide contents of hen ovalbumin, c-terminal sequence and location of disulfide bond. Biochem J 116:555–561
Gabler R (1978) Electrical interactions in molecular biophysics: an introduction. Academic Press Inc, London
Grade S, Eberhard J, Jakobi J, Winkel A, Stiesch M, Barcikowski S (2014) Alloying colloidal silver nanoparticles with gold disproportionally controls antibacterial and toxic effects. Gold Bull 47:83–93
Grdadolnik J (2002) Saturation effects in FTIR spectroscopy: intensity of amide I and amide II bands in protein spectra. Acta Chim Slov 50:777–788
Hu M, Chen J, Li ZY, Au L, Hartland GV, Li X, Marquez M, Xia Y (2006) Gold nanostructures: engineering their plasmonic properties for biomedical applications. Chem Soc Rev 35:1084–1094
Jiang X, Jiang J, Jin Y, Wang E, Dong S (2005) Effect of colloidal gold size on the conformational changes of adsorbed cytochrome c: probing by circular dichroism, UV–Visible, and infrared spectroscopy. Biomacromolecules 6:46–53
Jin X, Li M, Wang J, Marambio-Jones C, Peng F, Huang X, Damoiseaux R, Hoek EM (2010) High-throughput screening of silver nanoparticle stability and bacterial inactivation in aquatic media: influence of specific ions. Environ Sci Technol 44:7321–7328
Joshi D, Soni RK (2014) Laser-induced synthesis of silver nanoparticles and their conjugation with protein. Appl Phys A 116:635–641
Kabashin AV, Meunier M, Kingston C, Luong JHT (2003) Fabrication and characterization of gold nanoparticles by femtosecond laser ablation in an aqueous solution of cyclodextrins. J Phys Chem B 107:4527–4531
Kittler S, Greulich C, Gebauer JS, Diendorf J, Treuel L, Ruiz L, Gonzales-Calbet JM, Vallet-Regi M, Zellner R, Koller M, Epple M (2010) The influence of proteins on the dispersability and cell-biological activity of silver nanoparticles. J Mater Chem 20:512–518
Levin CS, Janesko BG, Bardhan R, Scuseria GE, Hartgerink JD, Halas NJ (2006) Chain-length-dependent vibrational resonances in alkanethiol self-assembled monolayers observed on plasmonic nanoparticle substrates. Nano Lett 6:2617–2621
Link S, El-Sayed MA (2000) Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. Int Rev Phys Chem 19:409–453
Mafune F, Kohno J, Takeda Y, Kondow T, Sawabe H (2000) Formation of gold nanoparticles by laser ablation in aqueous solution of surfactant. J Phys Chem B 105:5114–5120
Mafune F, Kohno J, Takeda Y, Kondow T (2001) Dissociation and aggregation of gold nanoparticles under laser irradiation. J Phys Chem B 105:9050–9056
Mutisya S, Franzela L, Barnsteinb BO, Faberb TW, Ryanb JJ, Bertinoa MF (2013) Comparison of in situ and ex situ bioconjugation of Au nanoparticles generated by laser ablation. Appl Surf Sci 264:27–30
Nakamura K, Era S, Ozaki Y, Sogami M, Hayashi T, Murakami M (1997) Conformational changes in seventeen cystine disulfide bridges of bovine serum albumin proved by Raman spectroscopy. FEBS Lett 417:375–378
Peng ZG, Hidajat K, Uddin MS (2004) Conformational change of adsorbed and desorbed bovine serum albumin on nano-sized magnetic particles. Colloids Surf B 33:15–21
Petersen S, Barcikowski S (2009) Conjugation efficiency of laser-based bioconjugation of gold nanoparticles with nucleic acids. J Phys Chem C 113:19830–19835
Ravindran A, Singh A, Raichur AM, Chandrasekaran N, Mukherjee A (2010) Studies on interaction of colloidal Ag nanoparticles with bovine serum albumin (BSA). Colloids Surf B 76:32–37
Roach P, Farrar D, Perry C (2006) Surface tailoring for controlled protein adsorption: effect of topography at the nanometer scale and chemistry. J Am Chem Soc 128:3939–3945
Sylvestre JP, Poulin S, Kabashin AV, Sacher E, Meunier M, Luong JHT (2004) Surface chemistry of gold nanoparticles produced by laser ablation in aqueous media. J Phys Chem B 108:16864–16869
Takahashi N, Tatsumi E, Orita T, Hirose M (2014) Role of the intrachain disulfide bond of ovalbumin during conversion into S-ovalbumin. Biosci Biotechnol Biochem 60:1464–1468
Takeda Y, Mafune F, Kondow T (2009) Selective degradation of proteins by laser irradiation onto gold nanoparticles in solution. J Phys Chem C113:5027–5030
Theodore P (1996) All about albumin: biochemistry, genetics and medical applications. Academic Press, San Diego
Tilaki RM, Irajizad A, Mahdavi SM (2006) Stability, size and optical properties of silver nanoparticles prepared by laser ablation in different carrier media. Appl Phys A 84:215–219
Tsuji T, Iryo K, Watanabe N, Tsuji M (2002) Preparation of silver nanoparticles by laser ablation in solution: influence of laser wavelength on particle size. Appl Surf Sci 202:80–85
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joshi, D., Soni, R.K. Synthesis of gold and silver nanoparticle S-ovalbumin protein conjugates by in situ conjugation process. J Nanopart Res 17, 210 (2015). https://doi.org/10.1007/s11051-015-3022-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-3022-5