Abstract
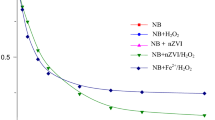
Emulsified nano-zero-valent iron (EZVI) is a modified form of bare nanoiron with improved transportability and targetability for the remediation of organic-solvents polluted soil and groundwater. In this work, EZVI (50–150 nm) was prepared by coating an emulsified vegetable oil membrane on the surface of Fe nanoparticles. EZVI was well-dispersed and less aggregation was observed. Batch experiments were conducted in anaerobic conditions to investigate the kinetics of nitrobenzene reduction by EZVI and the influences of oil concentration, initial iron content, and initial pH. Results indicated that the kinetics of nitrobenzene reduction by EZVI followed a pseudo-first-order kinetics. The observed rate constant of nitrobenzene is 0.0942 min−1. The oil concentration of 1 and 2 % tended to be preferred concentrations. The rate of nitrobenzene degradation and aniline formation increased with increasing iron content. The low pH is favorable to the nitrobenzene reduction by EZVI.














Similar content being viewed by others
References
Agrawal A, Tratnyek PG (1995) Reduction of nitro aromatic compounds by zero-valent iron metal. Environ Sci Technol 30:153–160
Berge ND, Ramsburg CA (2009) Oil-in-water emulsions for encapsulated delivery of reactive iron particles. Environ Sci Technol 43:5060–5066
Bezbaruah AN, Krajangpan S, Chisholm BJ, Khan E, Bermudez JJ (2009a) Entrapment of iron nanoparticles in calcium alginate beads for groundwater remediation applications. J Hazard Mater 166:1339–1343
Bezbaruah AN, Thompson JM, Chisholm BJ (2009b) Remediation of alachlor and atrazine contaminated water with zero-valent iron nanoparticles. J Environ Sci Health Part B Pestic Food Contam Agric Wastes 44:518–524
Bezbaruah AN, Shanbhogue SS, Simsek S, Khan E (2011) Encapsulation of iron nanoparticles in alginate biopolymer for trichloroethylene remediation. J Nanopart Res 13:6673–6681
Borden RC (2007a) Effective distribution of emulsified edible oil for enhanced anaerobic bioremediation. J Contam Hydrol 94:1–12
Borden RC (2007b) Concurrent bioremediation of perchlorate and 1,1,1-trichloroethane in an emulsified oil barrier. J Contam Hydrol 94:13–33
Borden RC, Lieberman T (2010) Edible oil barriers for treatment of chlorinated solvent and perchlorate-contaminated groundwater. Environmental Security Technology Certification Program Office (DOD), Arlington
Borden RC, Lieberman M T (2009) Passive bioremediation of perchlorate using emulsified edible oils. In Situ Bioremediation of Perchlorate in Groundwater 155-175
Chen JL, Al-Abed SR, Ryan JA, Li ZB (2001) Effects of pH on dechlorination of trichloroethylene by zero-valent iron. J Hazard Mater 83:243–254
Choe S, Liljestrand HM, Khim J (2004) Nitrate reduction by zero-valent iron under different pH regimes. Appl Geochem 19:335–342
Cohen M, Mercer JW, Matthews J (1993) DNAPL site evaluation. CK Smoley, Scranton
Dong J, Zhao Y, Zhao R, Zhou R (2010) Effects of pH and particle size on kinetics of nitrobenzene reduction by zero-valent iron. J Environ Sci 22:1741–1747
Dong J, Ding L, Wen C, Hong M, Jiang HZ (2013) Effects of geochemical constituents on the zero-valent iron reductive removal of nitrobenzene in groundwater. Water Environ J 27(1):20–28
Elliott DW, Zhang WX (2001) Field assessment of nanoscale bimetallic particles for groundwater treatment. Environ Sci Technol 35:4922–4926
Fagerlund F, Illangasekare T, Phenrat T, Kim HJ, Lowry GV (2012) PCE dissolution and simultaneous dechlorination by nanoscale zero-valent iron particles in a DNAPL source zone. J Contam Hydrol 131:9–28
Farrell J, Kason M, Melitas N, Li T (2000) Investigation of the long-term performance of zero-valent iron for reductive dechlorination of trichloroethylene. Environ Sci Technol 34:514–521
Hara SO, Krug T, Quinn J, Clausen C, Geiger C (2006) Field and laboratory evaluation of the treatment of DNAPL source zones using emulsified zero-valent iron. Remediat J 16:35–56
He F, Zhao D (2007) Manipulating the size and dispersibility of zerovalent iron nanoparticles by use of carboxymethyl cellulose stabilizers. Environ Sci Technol 41:6216–6221
Huang YH, Zhang TC (2006) Reduction of nitrobenzene and formation of corrosion coatings in zerovalent iron systems. Water Res 40:3075–3082
Johnson L, Nurmi JT, Johnson GSO, Fan D, Johnson RLO, Shi Z, Salter-Blane AJ, Tratnyek PG, Lowry GV (2013) Field-scale transport and transformation of carboxymethylcellulose-stabilized nano zero-valent iron. Environ Sci Technol 47:1573–1580
Kanel SR, Greneche JM, Choi H (2006) Arsenic(V) removal from groundwater using nano scale zero-valent iron as a colloidal reactive barrier material. Environ Sci Technol 40:2045–2050
Kim J, Tanapon P, Robert DT, Lowry GV (2012) Effect of kaolinite, silica fines and pH on transport of polymer-modified zero valent iron nano-particles in heterogeneous porous media. J Colloid Interface Sci 70:1–10
Laumann S, Micić V, Lowry GV, Hofmann T (2013) Carbonate minerals in porous media decrease mobility of polyacrylic acid modified zero-valent iron nanoparticles used for groundwater remediation. Environ Pollut 179:53–60
Li H, Zhao Y, Zhao R, Ma B, Chen Z, Su Y, Zhou R (2013) Characteristics and kinetics of nitrobenzene reduction by sucrose-modified nanoiron. Chem Res Chin Univ 29:765–770
Liu YQ, Lowry GV (2006) Effect of particle age (Fe0 content) and solution pH on NZVI reactivity: H2 evolution and TCE dechlorination. Environ Sci Technol 40:6085–6090
Liu Y, Phenrat T, Lowry GV (2007) Effect of TCE concentration and dissolved groundwater solutes on NZVI-promoted TCE dechlorination and H2 evolution. Environ Sci Technol 41:7881–7887
Mackay DM, Cherry JA (1989) Groundwater contamination: pump-and-treat remediation. Environ Sci Technol 23:630–636
Mackenzie K, Schierz A, Georgi A, Kopinke FD (2008) Colloidal activated carbon and carbo-iron-novel materials for in situ groundwater treatment. Glob NEST J 10:54–61
Manning BA, Hunt ML, Amrhein C, Yarmoff JA (2002) Arsenic(III) and arsenic(V) reactions with zerovalent iron corrosion products. Environ Sci Technol 36:5455–5461
Masciangioli T, Zhang WX (2003) Peer reviewed: environmental technologies at the nanoscale. Environ Sci Technol 37:102A–108A
Mu Y, Yu HQ, Zheng JC, Zhang SJ, Sheng GP (2004) Reductive degradation of nitrobenzene in aqueous solution by zero-valent iron. Chemosphere 54:789–794
Mueller NC, Braun J, Bruns J, Cernik M, Rissing P, Rickerby D, Nowack B (2012) Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe. Environ Sci Pollut Res 19:550–558
Nelson NT, Brusseau ML (1996) Field study of the partitioning tracer method for detection of dense nonaqueous phase liquid in a trichloroethene-contaminated aquifer. Environ Sci Technol 30:2859–2863
Phenrat T, Liu Y, Tilton RD, Lowry GV (2009a) Adsorbed polyelectrolyte coatings decrease Fe0 nanoparticle reactivity with TCE in water: conceptual model and mechanism. Environ Sci Technol 43:1507–1514
Phenrat T, Long TC, Lowry GV, Veronesi B (2009b) Partial oxidation (“aging”) and surface modification decrease the toxicity of nanosized zerovalent iron. Environ Sci Technol 43:195–200
Ponder SM, Darab JG, Mallouk TE (2000) Remediation of Cr(VI) and Pb(II) aqueous solutions using supported, nanoscale zero-valent iron. Environ Sci Technol 34:2564–2569
Quinn J, Geiger C, Clausen C, Brooks K, Coon C, Hara SO, Krug T, Major D, Yoon WS, Gavaskar A, Holdsworth T (2005) Field demonstration of DNAPL dehalogenation using emulsified zero-valent iron. Environ Sci Technol 39:1309–1318
Saleh N, Sirk K, Liu Y, Phenrat T, Dufour B, Matyjaszewski K, Tilon RD, Lowry GV (2007) Surface modifications enhance nanoiron transport and NAPL targeting in saturated porous media[J]. Environ Eng Sci 24:45–57
Scherer MM, Johnson KM, Westall JC, Tratnyek PG (2001) Mass transport effects on the kinetics of nitrobenzene reduction by iron metal. Environ Sci Technol 35:2804–2811
Schrick B, Hydutsky BW, Blough JL, Mallouk J (2004) Delivery vehicles for zerovalent metal nanoparticles in soil and groundwater. Chem Mater 16:2187–2193
Sita K, Harjyoti K, Bret JC, Bezbaruah AN (2012) Iron nanoparticles coated with amphiphilic polysiloxane graft copolymers: dispersibility and contaminant treatability. Environ Sci Technol 46:10130–10136
Sohn K, Kang SW, Ahn S, Woo M, Yang SK (2006) Fe(0) nanoparticles for nitrate reduction: stability, reactivity, and transformation. Environ Sci Technol 40:5514–5519
Suk OJ, Jeen SW, Gillham RW, Gui L (2009) Effects of initial iron corrosion rate on long-term performance of iron permeable reactive barriers: column experiments and numerial simulation. J Contam Hydrol 103:45–156
Wang CB, Zhang WX (1997) Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs. Environ Sci Technol 31:2154–2156
Wang W, Zhou M (2010) Degradation of trichloroethylene using solvent-responsive polymer coated Fe nanoparticles. Colloids Surf A 369:232–239
Yang GCC, Tu HC, Hung CH (2007) Stability of nano iron slurries and their transport in the subsurface environment. Sep Purif Technol 58:166–172
Zhang WX (2003) Nanoscale iron particles for environmental remediation: an overview. J Nanopart Res 5:323–332
Zhang W, Elliott DW (2006) Applications of iron nanoparticles for groundwater remediation. Remediat J 16:7–21
Zhu H, Jia Y, Wu X, Wang H (2009) Removal of arsenic from water by supported nano zero-valent iron on activated carbon. J Hazard Mater 172:1591–1596
Acknowledgments
This work was supported by Key Laboratory of Groundwater Resources and Environment, Ministry of Education; National Natural Science Foundation (No. 41272253); Jilin Province Natural Science Foundation (No. 20130101027JC).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dong, J., Wen, C., Liu, D. et al. Study on degradation of nitrobenzene in groundwater using emulsified nano-zero-valent iron. J Nanopart Res 17, 31 (2015). https://doi.org/10.1007/s11051-014-2829-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2829-9