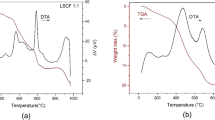
Abstract
Nanopowders of La0.75Sr0.25Cr0.5Mn0.5O3−δ (LSCM) perovskite-type oxide, potential electrode material for symmetrical solid oxide fuel cell (S-SOFC), have been successfully prepared by the solution combustion method employing glycine as complexing agent and fuel. Several glycine-to-nitrates molar ratios were investigated. A detailed morphological and structural characterization was performed, employing X-ray diffraction, N2 physisorption, and electron microscopy (scanning and transmission). The as-synthesized LSCM nanopowders consist of interconnected nanoparticles forming a sponge-like structure with typical meso- and macropores. For glycine-to-nitrates molar ratios above stoichiometric molar ratio, a high proportion of well-crystallized LSCM phase was obtained. An increase of glycine content in the initial synthesis gel, decreases the average nanoparticles size from ~20 to ~6 nm, narrows the distribution crystallite size, and increases the specific surface area of the LSCM nanopowders from ~14 to ~30 m2/g. The evolution of crystalline phases of the as-synthesized LSCM nanopowders after calcination at various temperatures was studied: pure-phase La0.75Sr0.25Cr0.5Mn0.5O3−δ perovskites with particle-size distribution between 100 and 300 nm, have been obtained after calcination at 1,000 °C for 6 h. The crystalline structural analyses showed that the LSCM nanocrystallites have trigonal/rhombohedral symmetry in the R-3c space group. These final LSCM nanopowders present microstructures with low densification and open porosity but probably with sufficient integrity to enable them to be used as efficient electrodes in S-SOFC.








Similar content being viewed by others
References
Bastidas DM, Tao S, Irvine JTS (2006) A symmetrical solid oxide fuel cell demonstrating redox stable perovskite electrodes. J Mater Chem 16:1603–1605
Bates JL, Chick LA, Weber WJ (1992) Synthesis, air sintering and properties of lanthanum and yttrium chromites and manganites. Solid State Ionics 52:235–242
Chick LA, Pederson LR, Maupin GD, Bates JL, Thomas LE, Exarhos GJ (1990) Glycine-nitrate combustion synthesis of oxide ceramic powders. Mater Lett 10:6–12
Civera A, Pavese M, Saracco G, Specchia V (2003) Combustion synthesis of perovskite-type catalysts for natural gas combustion. Catal Today 83:199–211
Delahaye T, Jardiel T, Joubert O, Laucournet R, Gauthier G, Caldes MT (2011) Electrochemical properties of novel SOFC dual electrode La0.75Sr0.25Cr0.5Mn0.3Ni0.2O3−δ. Solid State Ionics 184:39–41
Estemirova S, Fetisov A, Balakirev V, Titova SJ (2007) Crystal structure and magnetic properties of (La1−x Sr x MnO3) N (LaCrO3)1−N solid solutions. Supercond Nov Magn 20:113–116
Finger LW, Cox DE, Jephcoat AP (1994) A correction for powder diffraction peak asymmetry due to axial divergence. J Appl Crystallogr 27:892–900
Ha SB, Cho PS, Cho YH, Lee D, Lee JH (2010) Preparation of La0.75Sr0.25Cr0.5Mn0.5O3−δ fine powders by carbonate coprecipitation for solid oxide fuel cells. J Power Sources 195:124–129
Jain SR, Adiga KC, Pai Verneker VR (1981) A new approach to thermochemical calculations of condensed fuel-oxidizer mixtures. Combust Flame 40:71–79
Jiang SP, Zhang L, Cheng CS, Zhang Y (2007a) Development of (La0.75Sr0.25)(Cr0.5Mn0.5)O3 cathodes of solid oxide fuel cells by gelcasting technique. ECS Trans 7:1081–1088
Jiang SP, Zhang L, Zhang Y (2007b) Lanthanum strontium manganese chromite cathode and anode synthesized by gel-casting for solid oxide fuel cells. J Mater Chem 17:2627–2635
Minh NQ, Takahashi T (1995) Science and technology of ceramic fuel cells. Elsevier, Amsterdam
Najjar H, Batis H (2010) La–Mn perovskite-type oxide prepared by combustion method: catalytic activity in ethanol oxidation. Appl Catal A 383:192–201
Najjar H, Lamonier JF, Mentré O, Giraudon JM, Batis H (2011) Optimization of the combustion synthesis towards efficient LaMnO3+y catalysts in methane oxidation. Appl Catal B 106:149–159
Perez-Falcon JM, Moure A, Tartaj J (2011) Low-temperature preparation of La0.6Sr0.4Fe0.8Co0.2O3–δ sinterable nanopowders by the polymeric organic complex solution method. Fuel Cells 11:75–80
Raj ES, Kilner JA, Irvine JTS (2006) Oxygen diffusion and surface exchange studies on (La0.75Sr0.25)0.95Cr0.5Mn0.5O3−δ. Solid State Ionics 177:1747–1752
Rietveld HM (1969) A profile refinement method for nuclear and magnetic structures. J Appl Crystallogr 2:65–71
Rodríguez-Carvajal J (1993) Recent advances in magnetic structure determination by neutron powder diffraction. Phys B: Cond Matter 192:55–69
Ruiz-Morales JC, Canales-Vázquez J, Peña-Martínez J, Marrero López D, Núñez P (2006) On the simultaneous use of La0.75Sr0.25Cr0.5Mn0.5O3−δ as both anode and cathode material with improved microstructure in solid oxide fuel cells. Electrochim Acta 52:278–284
Ruiz-Morales JC, Canales-Vázquez J, Lincke H, Peña-Martínez J, Marrero-López D, Pérez-Coll D, Irvine JTS, Núñez P (2008) Potential electrode materials for symmetrical solid oxide fuel cells. Bol Soc Esp Ceram Vidr 47:183–188
Shao Z, Zhou W, Zhu Z (2012) Advanced synthesis of materials for intermediate-temperature solid oxide fuel cells. Progr Mater Sci 57:804–874
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 57:603–619
Singhal SC, Kendall K (2004) High temperature solid oxide fuel cells. Elsevier, Oxford
Tao S, Irvine JTS (2003) A redox-stable, efficient anode for solid-oxide fuel cells. Nat Mater 2:320–323
Tao S, Irvine JTS (2004) Synthesis and characterization of (La0.75Sr0.25)Cr0.5Mn0.5O3–, a redox-stable, efficient perovskite anode for SOFCs. J Electrochem Soc 151:A252–A259
Tao S, Irvine JTS (2006) Phase transition in perovskite oxide La0.75Sr0.25Cr0.5Mn0.5O3-δ observed by in situ high-temperature neutron powder diffraction. Chem Mater 18:5453–5460
Toniolo JC, Lima MD, Takimi AS, Bergmann CP (2005) Synthesis of alumina powders by the glycine–nitrate combustion process. Mater Res Bull 40:561–571
Traina K, Steil MC, Pirard JP, Henrist C, Rulmont A, Cloots R, Vertruyen B (2007) Synthesis of La0.9Sr0.1Ga0.8Mg0.2O2.85 by successive freeze-drying and self-ignition of a hydroxypropylmethyl cellulose solution. J Eur Ceram Soc 27:3469–3474
Villoria JA, Alvarez-Galvan MC, Al-Zahrani SM, Palmisano P, Specchia S, Specchia V, Fierro JLG, Navarro RM (2011) Oxidative reforming of diesel fuel over LaCoO3 perovskite derived catalysts: influence of perovskite synthesis method on catalyst properties and performance. Appl Catal B 105:276–288
Young RA (1996) The rietveld method. International Union of Crystallography & Oxford University Press, New York
Zha S, Tsang P, Cheng Z, Liu M (2005) Electrical properties and sulfur tolerance of La0.75Sr0.25Cr1−xMnxO3 under anodic conditions. J Solid State Chem 178:1844–1850
Zhua X, Lüa Z, Wei B, Huang X, Zhang Y, Su W (2011) A symmetrical solid oxide fuel cell prepared by dry-pressing and impregnating methods. J Power Sources 196:729–733
Acknowledgments
This study was supported by the CONICET and CAB-CNEA of Argentina. C.M.C. thanks Eng. Luisa Fernández Albanesi and Dr. Fabiana Gennari for the N2 adsorption measurements.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chanquía, C.M., Vega-Castillo, J.E., Soldati, A.L. et al. Synthesis and characterization of pure-phase La0.75Sr0.25Cr0.5Mn0.5O3−δ nanocrystallites for solid oxide fuel cell applications. J Nanopart Res 14, 1104 (2012). https://doi.org/10.1007/s11051-012-1104-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-1104-1