Abstract
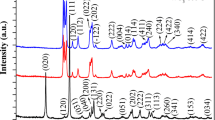
Monodisperse α-Fe2O3 microspheres have been selectively synthesized through a facile hydrothermal method without the assistance of any surfactant, employing FeCl3·6H2O and NH4NaHPO4 as initial materials. The products were characterized by X-ray diffraction, scanning electron microscopy, and transmission electron microscopy. α-Fe2O3 microspheres with average size about 250 nm were constructed by single crystalline nanoparticles with average diameter about 15 nm. The investigation on the evolution formation revealed that growth temperature was critical to control the assembly of the fresh formed nanocrystallites, and the microsphere formation was proved to be the Ostwald ripening process by tracking the structures of the products at different growth temperature. α-Fe2O3 microspheres showed a weak ferromagnetic behavior with a remanent magnetization of 0.208 emu g−1 and a coercivity of 1,034.27 Oe at room temperature.








Similar content being viewed by others
References
Cao MH, Liu TF, Gao S, Sun GB, Wang XL, Hu CW, Wang ZL (2005) Single-crystal dendritic micro-pines of magnetic a-Fe2O3: large-scale synthesis, formation mechanism, and properties. Angew Chem Int Ed 44:4197–4201
Cao HQ, Wang GZ, Zhang L, Liang Y, Zhang SC, Zhang XR (2006) Shape and magnetic properties of single-crystalline hematite α-Fe2O3 nanocrystals. Chem Phys Chem 7(9):1897–1901
Chaudhari NK, Kim HC, Son D, Yu JS (2009) Easy synthesis and characterization of single-crystalline hexagonal prism-shaped hematite α-Fe2O3 in aqueous media. Cryst Eng Comm 11:2264–2267
Cole JJ, Wang X, Knuesel RJ, Jacobs HO (2008) Integration of ZnO microcrystals with tailored dimensions forming light emitting diodes and UV photovoltaic cells. Nano Lett 8(5):1477–1481
Cornell RM, Schwertmann U (2003) The iron oxides: structure, properties, reactions, occurrences and uses. Wiley, Weinheim
Flynn CM Jr (1984) Hydrolysis of inorganic iron (III) salts. Chem Rev 84:31–41
Ganguli AK, Ahmad T (2007) Nanorods of iron oxalate synthesized using reverse micelles: facile route for α-Fe2O3 and Fe3O4 nanoparticles. J Nanosci Nanotechn 7(6):2029–2035
Hao R, Xing RJ, Xu ZC, Hou YL, Gao S, Sun SH (2010) Synthesis, functionalization and biomedical applications of multifunctional magnetic nanoparticles. Adv Mater 22:2729–2742
Hu XL, Yu JC (2008) Continuous aspect-ratio tuning and fine shape control of monodisperse α-Fe2O3 nanocrystals by a programmed microwave–hydrothermal method. Adv Funct Mater 18:880–887
Li J, Zeng HC (2007) Hollowing Sn-doped TiO2 nanospheres via Ostwald ripening. J Am Chem Soc 129(51):15839–15847
Li LL, Chu Y, Liu Y, Dong LH (2007) Template-free synthesis and photocatalytic properties of novel α-Fe2O3 hollow spheres. J Phys Chem C 111:2123–2127
Liu XM, Fu SY, Xiao HM, Huang CJ (2005a) Preparation and characterization of shuttle-like@α-Fe2O3 nanoparticles by supermolecular template. J Solid State Chem 178(9):2798–2803
Liu XM, Fu SY, Xiao HM, Huang CJ (2005b) Preparation and characterization of shuttle-like α-Fe2O3 nanoparticles by supermolecular template. J Solid State Chem 178:2798–2803
Ma M, Zhan YQ, Shen YQ, Xia X, Zhang SM, Liu ZL (2011) Synthesis of amino-group functionalized superparamagnetic iron oxide nanoparticles and applications as biomedical labeling probes. J Nanopart Res 13:3249–3257
Nam KT, Kim DW, Yoo PJ, Chiang CY, Meethong N, Hammond PT et al (2006) Virus-enabled synthesis and assembly of nanowires for lithium ion battery electrodes. Science 312(5775):885–888
Oh JY, Park J, Kang SY, Hwang CS, Shim HK (2009) Room temperature fabrication of ZnO nanorod films: synthesis and application as a channel layer of transparent thin film transistors. Chem Commun 30:4545–4547
Sugimoto T (1987) Preparation of monodispersed colloidal particles. Adv Colloid Interface Sci 28:65–108
Tang B, Wang GL, Zhuo LH, Ge JC, Cui L (2006) Facile route to α-FeOOH and α-Fe2O3 nanorods and magnetic property of α-Fe2O3 nanorods. Inorg Chem 45:5196–5200
Wang DB, Song CX, Zhao YH, Yang ML (2008a) Synthesis and characterization of monodisperse iron oxides microspheres. J Phys Chem C 112:12710–12715
Wang DB, Song CX, Zhao YH, Yang ML (2008b) Synthesis and characterization of monodisperse iron oxides microspheres. J Phys Chem C 112(33):12710–12715
Xuan SH, Hao LY, Jiang WQ, Song L, Hu Y, Chen ZY et al (2007) A FeCO3 precursor-based route to microsized peanutlike Fe3O4. Cryst Growth Des 7(2):430–434
Yang WH, Lee CF, Tang HY, Shieh DB, Yeh CS (2006) Iron oxide nanopropellers prepared by a low temperature solution approach. J Phys Chem B 110:14087–14091
Yu XL, Cao CB, An XQ (2008) Facile conversion of Fe nanotube arrays to novel α-Fe2O3 nanoparticle nanotube arrays and their magnetic properties. Chem Mater 20:1936–1940
Zhu LP, Xiao HM, Liu XM, Fu SY (2006) Template-free synthesis and characterization of novel 3D urchin-like α-Fe2O3 superstructures. J Mater Chem 16:1794–1797
Zhu LP, Xiao HM, Fu SY (2007) Template-free synthesis of monodispersed and single-crystalline cantaloupe-like Fe2O3 superstructures. Cryst Growth Des 7(2):177–182
Acknowledgments
This work has been supported by the National Nature Science Foundation (50903040, 51103065), the special grade of the financial support from China Postdoctoral Science Foundation (201003554), the China Postdoctoral Science Foundation (20090451169), the Jiangsu Postdoctoral Science Foundation (0901078C), the Jiangsu Key Lab of material tribology Foundation (kjsmcx0905), and the Senior Intellectuals Foundation of Jiangsu University (09JDG003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jia, XH., Song, HJ. Facile synthesis of monodispersed α-Fe2O3 microspheres through template-free hydrothermal route. J Nanopart Res 14, 663 (2012). https://doi.org/10.1007/s11051-011-0663-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-011-0663-x