Abstract
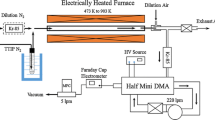
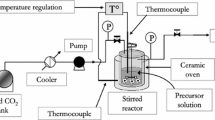
Thermal decomposition of titanium tetraisopropoxide (TTIP) was carried out in varying reaction atmospheres: nitrogen, oxygen, and nitrogen plus water vapor. The effect of reaction atmosphere on the morphology, size, and crystalline structure of produced TiO2 particles was studied. The reactor used was similar to the microreactor proposed earlier by Park et al. (2001, J. Nanopart. Res., 3, 309–319), but for a modification in the precursor evaporator. The reactor temperature was varied from 300 to 700°C and the TTIP concentration in the evaporator from 1.0 to 7.0 mol%, holding the reactor residence time at 0.7 s. The primary-particle size was in the range 25–250 nm, varying with operating condition. The crystalline structure was amorphous in nitrogen, a mixture of rutile and anatase in nitrogen plus water vapor, and anatase in oxygen atmospheres. In nitrogen, agglomerates composed of very small particles whose individual boundaries are not clearly distinguished were produced. In oxygen, the particles composing an agglomerate became larger and were clearly spherical. As the atmosphere was varied to the nitrogen plus water vapor, the particle size increased further. The variation of primary particle size with reaction atmosphere was discussed in comparison with previous experimental data.
Similar content being viewed by others
References
Ahonen P.P., Moisala A., Tapper U., Brown D.P., Jokiniemi J.K. and Kauppinen E.I., (2002). Gas-phase crystallization of titanium dioxide nanoparticles. J. Nanopart. Res. 4: 43–42
Akhtar M.K., Vemury S. and Pratsinis S.E., (1994). Competition between TiCl4 Hydrolysis and Oxidation and its Effect on Product TiO2 powder. AIChE J. 40: 1183–1192
Alam M.K. and Flagan R.C., (1986). Controlled nucleation aerosol reactors: Production of bulk silicon. Aerosol Sci. Technol. 5: 237–248
Kirkbir F. and Komiyama H., (1987). Formation and growth mechanism of porous, amorphous, and fine particles prepared by chemical vapor deposition. Titania from titanium tetraisopropoxide. Can. J. Chem. Eng. 65: 759–766
Kirkbir F. and Komiyama H., (1988). Continuous production of fine TiO2 powders by vapor-phase hydrolysis of titanium tetraisopropoxide. Advanced Ceramic Materials. 3: 511–515
Komiyama H., Kanai T. and Inoue H., (1984). Preparation of porous, amorphous, and ultrafine TiO2 particles by chemical vapor deposition. Chem. Lett. 152: 1283–1286
Moravec P., Smolik J. and Levdansky V.V., (2001). Preparation of TiO2fine particles by thermal decomposition of titanium tetraisopropoxide vapor. J. Mater. Sci. Lett. 20: 2033–2037
Okuyama K., Kousaka Y., Tohge N., Yamamoto S., Wu J.J., Flagan R.C. and Seinfeld J.H., (1986). Production of ultrafine metal oxide aerosol particles by thermal decomposition of metal alkoxide vapors. AIChE J 32: 2010–2019
Park K.Y., Ullmann M., Suh Y.J. and Friedlander S.K., (2001). Nanoparticle microreactor: Application to synthesis of titania by thermal decomposition of titanium tetraisoproxide. J. Nanopart. Res. 3: 309–319
Park K.Y., Jang H.D. and Choi C.S., (1991). Generation of ultrafine iron powders by gas-phase reduction of ferrous chloride with hydrogen. J. Aerosol Sci. 22: S113-S116
Satake T., T. Sorita & H. Adachi, 1996. Kinetic and theoretical study of the thermal decomposition of tetraethoxysilane in the gas phase. In: Besmann T.M., Allendorf M.D., Robinson Mc D. & Ulrich R.K. eds. Proceedings of the Thirteenth International Conference on Chemical Vapor Deposition, Los Angeles, CA, USA, 5–10 May, pp. 23–28
Schleich D.M. and Walter B., (1997). Formation of titania nanoparticles by vapor phase reactions of titanium tetraisopropoxide in oxygen/ozone containing atmospheres. Nanostruct. Mater. 8: 579–586
Suyama Y. and Kato A., (1976). TiO2 produced by vapor-phase oxygenolysis of TiCl4. J. Am. Ceram. Soc. 59: 146–149
van der Vis M.G.M., Cordfunke E.H.P. and Konings R.J.M., (1993). The thermodynamic properties of tetraethoxysilane (TEOS) and an infrared study of its thermal decomposition. J. Phys. IV, Colloque C3: 75–82
Acknowledgment
This work was supported by the Korea Research Foundation Grant (KRF-2004-041-D00195).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, J.G., Park, K.Y. Effect of reaction atmosphere on particle morphology of TiO2 produced by thermal decomposition of titanium tetraisopropoxide. J Nanopart Res 8, 269–278 (2006). https://doi.org/10.1007/s11051-005-9042-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-005-9042-9