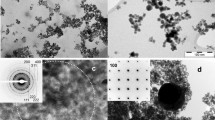
Abstract
In this study a process for removing the protective encapsulate from salt encapsulated nanopowders has been developed and tested for tantalum nanopowder produced by sodium/halide gas-phase combustion synthesis. A sodium/halide flame can be used to produce salt-encapsulated nanoparticles for many metal and nonoxide ceramic materials. The salt encapsulate allows for control of size and morphology, and protects the core particles from oxygen contamination. Without a protective coating, non-oxide nanopowders can be pyrophoric or form a surface oxide. Despite the beneficial attributes of the NaCl encapsulate, it can also be a source of contamination. Thus, a method by which the encapsulate can be removed and the exposed particles subsequently processed without exposure to environmental contamination was developed. A two step process was developed, where the bulk of the salt is sublimed from the core particles at 880°C and directed out of the system with an inert gas flow followed by a vacuum sublimation at temperatures at or greater than 1200°C for 120 min. These conditions reduced Na and Cl concentrations to below detectable limits of their respective analyses (5 ppm Na and 20 ppm Cl). Heat treating the core particles for 120 min at 1200°C, 1400°C and 1600°C coarsened the particles from 30 nm to approximately 250 nm, 400 nm and 3µm, respectively. At 1600°C a fully dense Ta consolidate was produced by applying a uniaxial load of 45 MPa pressure for 210 min.
Similar content being viewed by others
References
Andrievski R.A. (1994). Compaction and sintering of ultrafine powders. Int. J. Powder Metall. 30(1): 59–66
Axelbaum R.L. (2000). Synthesis of stable metal and non-oxide ceramic nanoparticles in sodium/halide flames. Powder Metall. 43(4): 323–325
Axelbaum, R.L., DuFaux, D.P. & Rosen, L.J., 1995. Method and Apparatus for Producing High Purity and Unagglomerated Submicron Particles. U.S. Patent No. 5498446
Axelbaum R.L., Lottes C.R., Huertas J.I. and Rosen L.J. (1996). Gas-phase combustion synthesis of aluminum nitride powders. Proc. Combust. Inst. 26: 1891–1897
Cho H., Waters M.A. and Hogg R. (1996). Investigation of the grind limit in stirred-media milling. Int. J. Mineral Proc. 44–45: 607–615
Cox, D.M., 1999. WTEC Panel Report on Nanostructure Science and Technology: R & D Status and Trends in Nanoparticles, Nanostructured Materials, and Nanodevices: Final Report: December 1998. 49–66
DuFaux D.P. and Axelbaum R.L. (1995). Nanoscale unagglomerated nonoxide particles from a sodium coflow flame. Combust. Flame 100: 350–358
Gill J. (1996). Basic Tantalum Capacitor Technology. AVX Technical Information Articles. AVX Corporation, England
Groat E.A. and Mrox T.J. (1994). Aqueous slip casting of stabilized AIN powders. Am. Ceramic Soc. Bull. 73(11): 75–78
Groza J.R. and Dowding R.J. (1996). Nanoparticulate materials densification. Nanostruct. Mater. 7(7): 749–768
Haber J.A. and Buhro W.E. (1998). Kinetic instability of nanocrystalline aluminum prepared by chemical synthesis; Facile room-temperature grain growth. J. Am. Chem. Soc. 120(42): 10847–10855
Hirsch P.B., Howie A., Nicholson R.B., Pashley D.W. and Whelan M.J. (1965). Electron Microscopy of Thin Crystals. Butterworths, London
Jena P., Khanna S.N. and Rao B.K. (1996). Stability and electronic structure of cluster assembled materials. Mater. Sci. Forum 232: 1–25
Kaufmann D.W. (1960). Sodium Chloride, The Production and Properties of Salt and Brine. Reinhold Publishing Corp, New York
Morris D.G. and Morris M.A. (1997). Hardness, strength, ductility and toughness of nanocrystalline materials. Mater. Sci. Forum 235–238: 861–872
Moser K.D. (1999). The manufacture and fabrication of tantalum. J. Minerals Metals Mater. Soc. 51(4): 29–31
Shriver D.F. and Drezdzon M.A. (1986.). The Manipulation of Air-Sensitive Compounds. , JohnWiley & Sons, New York
Suryanarayana, C. & Norton, M.G., 1998. X-Ray Difrraction: A Practical Approach. Plenum Publishing Corporation, pp. 207–221
Weissmuller, J., 1996. Nanocrystalline Materials, an Overview. The Minerals Metals, and Materials Society, pp. 3–19
Zhang H., Penn R.L., Hamers R.J. and Banfield J.F. (1999). Enhanced adsorption of molecules on surfaces of nanocrystalline particles. J. Phys. Chem. B 103(22): 4656–4662
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barr, J.L., Axelbaum, R.L. & Macias, M.E. Processing Salt-encapsulated Tantalum Nanoparticles for High Purity, Ultra High Surface Area Applications. J Nanopart Res 8, 11–22 (2006). https://doi.org/10.1007/s11051-005-8336-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-005-8336-2