Abstract
Exophiala spp. is increasingly reported as a pathogen causing the cutaneous, subcutaneous or invasive infection. In this report, we present a case of cutaneous phaeohyphomycosis due to E. jeanselmei on the right hand of a farmer, who suffered from this disease three years ago which had not been definitely diagnosed until he was admitted to our hospital. In our hospital, a potential fungal pathogen was observed by histopathological examination, and then was recovered and identified as E. jeanselmei by sequencing its internal transcribed spacer region. After 4 weeks of antifungal treatment, his hand recovered very well. To investigate the in vitro susceptibility of E. jeanselmei isolates to antifungal agents and compare the characteristics of their related infections among immunocompetent and immunocompromised patients, we reviewed 84 cases published in PubMed database between 1980 and 2020.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phaeohyphomycosis is a group of rare fungal infections caused by the dematiaceous fungi, such as Alternaria spp. Phialophora spp. and Exophiala spp. [1]. Recently, Exophiala spp. has been frequently reported as an etiologic agent of phaeohyphomycosis in both immunocompromised and immunocompetent individuals [2,3,4,5], which highlights the importance of reviewing the characteristics of this pathogen related infections. In this study, we described one case of prolonged cutaneous infection due to E. jeanselmei and reviewed 84 cases published in PubMed database during 1980 to 2020.
Case Report
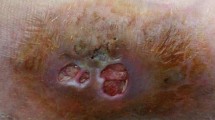
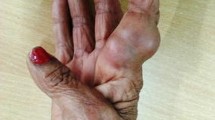
A 61-year-old male farmer was admitted to West China Hospital of Sichuan University on October 30, 2018. There were multiple cutaneous abscesses on the back of the red, swollen and painful right hand (Fig. 1a). Computed tomography (CT) scan of this hand showed the extensive tissue swelling in the wrist and palm (Fig. 1b).
The presentation of the right hand before and after antifungal treatment in our case. a There were multiple cutaneous abscesses on the back of the red, swollen and painful right hand when the patient was admitted to our hospital. b Computed tomography scan of the hand showed the extensive tissue swelling in the wrist and palm. c After one week treatment with amphotericin B, the pus no longer discharged and the skin began to scab. d Voriconazole was administered for the patient after the isolate was identified as E. jeanselmei, the hand recovered very well after another two weeks
He complained that this hand was injured when he worked in the field three years ago. And then the swelling and pain was developed, while the definite diagnosis was not made in the local hospital and triamcinolone acetonide was administrated intermittently to relieve the discomfort of this hand. Two months ago, the old skin lesions in this hand expanded rapidly and new ones appeared. Some kinds of the antibiotics were administered in the local hospital but the symptoms did not improve. He denied the history of autoimmune diseases, diabetes and tumors.
At his admission to our hospital, white blood cell count (7.27 × 109/L), neutrophilic granulocyte ratio (85.3%), serum C-reactive protein (20.76 mg/L) and procalcitonin (0.22 ng/mL) were all elevated. The skin biospy of this hand was sent for pathological investigation. Multiple dark hyphae were highlighted with Gomori methenamine silver (GMS) staining (Fig. 2a).
The positive results of the specimens for pathogenic examination in our case. a Multiple dark hyphae were highlighted with Gomori methenamine silver (GMS) staining of skin biopsy as the arrows point to. b Flat to domed, mucoid, dark olive-green to black colonies with black reverse growed at 28 °C on Sabouraud’s dextrose agar (SDA) after one week incubation. c The septate hyphae, conidia, and annellides ware stained with Lactophenol cotton blue for the isolate incubated on SDA plate for one week. d The septate hyphae, conidia, and annellides ware stained with Lactophenol cotton blue for the isolate incubated on Potato dextrose agar (PDA) plate for three weeks
Thick brown aspirate collected by fine-needle was sent for bacterial, fungal, and mycobacterial investigation. The results of Gram staining and acid-fast staining were negative. There were flat to domed, mucoid, dark olive-green to black colonies with black reverse at 28 °C on sabouraud’s dextrose agar (SDA) plate (Fig. 2b). The colonies were stained with lactophenol cotton blue. The septate hyphae, single-celled and ellipsoidal conidia accumulating in groups, and cylindrical to flask-shaped annellides with narrow apical area, were observed (Fig. 2c, 2d).
Isolation of Exophiala spp. from the aspirate was reported to the clinicians. This patient was administered with amphotericin B (1 mg/kg iv q12h) immediately. After one week, the pus no longer discharged and the skin began to scab (Fig. 1c).
Sequencing the internal transcribed spacer (ITS) was performed to identify the isolate to species level as previously reported [6], and its sequence were completely homology with those of E. jeanselmei D12H (Accession ID: MH010928.1).
In vitro susceptibility testing of this isolate to antifungal agents was performed according to the M38-A2 protocol of Clinical and Laboratory Standards Institute (CLSI) [7]. Briefly, the conidia was collected with a cotton swab and suspended in sterile saline with Tween. Heavy particles were settled for 3 to 5 min. The supernatant was collected and mixed with a vortex mixer. Its turbidity was adjusted to a 0.5 McFarland Standard. And then, 100 μL of the suspension was added to 11 mL of Sensititre YeastOne broth (Thermo Fisher Co.) to give a final inoculum. The final suspension was transferred into the Sensititre YeastOne plate and incubated at 35 °C in a non-CO2 incubator. The minimum inhibitory concentrations (MICs) were reported as listed in Table 1. Voriconazole (200 mg iv q12h) was administered for this patient. One week later, he was discharged with oral voriconazole (200 mg, bid). After another two weeks, his hand recovered very well (Fig. 1d).
Literature Review of the Cases Associated with E. jeanselmei
A total of 89 cases associated with E. jeanselmei during 1980 to 2020 were collected from PubMed database (http://www.ncbi.nlm.nih.gov/pubmed), but five of them were excluded for lack of detailed data [2,3,4, 6, 8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92]. We summarized the available results of in vitro susceptibility testing against 70 strains of E. jeanselmei described in Table 1. Except one study described the E-test method [39], broth microdilution method were performed in the other reports. The antifungal agents tested frequently were amphotericin B, azoles and echinocandins. In addition, two studies tested the activity of terbinafine [38, 59].
The 84 cases included were classified into immunocompetent or immunocompromised group according to their underlying conditions. The clinical characteristics of the infections among these two groups were compared as shown in Table 2.
Discussion
Phaeohyphomycosis is a rare infection caused by the dematiaceous fungi [93]. In recent years, the frequent occurrence of this kind of fungal infection as well as the diversity of the organisms isolated has been reported around the world [94, 95]. Alternaria spp. was associated with the cutaneous phaeohyphomycosis in solid-organ transplant recipients [89, 96, 97]. Scedosporium prolificans was a common species (41.6%) responsible for disseminated phaeohyphomycosis [98]. E. jeanselmei was involved in the subcutaneous or cutaneous infections [65, 99]. Exophiala spp. was isolated from 80.0% of the cases had underlying collagen disease and E. jeanselmei was the most common species (46.2%) [2]. In this study, we shared an experience of successful diagnosis and treatment of a prolonged cutaneous phaeohyphomycosis due to E. jeanselmei, and also reviewed the cases associated with this species.
Accurate identification of the pathogen can provide an important basis for precise diagnosis and treatment of phaeohyphomycosis. Otherwise, prolonged disease process, disseminated or relapsed situation, even poor clinical outcomes might be brought about [100, 101]. Traditionally, histopathological examination and culture-based methods are applied to identify the fungal pathogen. Whereas, these methods are usually time-consuming and confused morphological characteristics might bring the challenges for the inexperienced technicians. Therefore, molecular-based methods are preferred especially when typical morphological characteristics cannot be observed. In our case, the internal transcribed spacer (ITS) regions of the isolate were sequenced. Eventually, a cutaneous phaeohyphomycosis associated with E. jeanselmei was diagnosed based on the clinical features, histopathological examination, culture and ITS sequencing of the isolate.
In our study, in vitro susceptibility of E. jeanselmei isolates to antifungal agents were also reviewed. As shown in Table 1, this species had good in vitro susceptibility to amphotericin B, posaconazole, voriconazole, itraconazole and echinocandins. Variable activity with wide MIC range of amphotericin B (0.25–16 µg/mL), voriconazole (0.03–4 µg/mL) and itraconazole (< 0.015–64 µg/mL) were also observed in Table 1. In practice, susceptibility testing for each isolate should be performed to guide the precise treatment. In addition, other factors should be considered when the agents were chosen. Amphotericin B was a potent broad-spectrum antifungal drug for fungal infections, but it is not the best choice due to severe side effects [102]. Azoles were reported as the active drugs against E. jeanselmei [103].Though itraconazole showed significant activity against dematiaceous fungi, adverse effects and the lack of an intravenous formulation have reduced its use [99, 104]. Voriconazole was likely a good choice due to its broad activity, preferable side effect profile, and availability of an intravenous formulation [105]. Echinocandins had a variable in vitro activity and were not suggested by the guidelines of European Society for Clinical Microbiology and Infectious Diseases and European Confederation of Medical Mycology (ESCMID/ECMM) [102]. Terbinafine was also the treatment option for cutaneous and subcutaneous infection [38]. The lack of serious side effects and broad-spectrum in vitro antifungal activity help make terbinafine an alternative drug for the patients who cannot be cured through conventionally recommended treatments [106].
According to ESCMID/ECMM guidelines, antifungal therapy combination with surgical excision was recommended for managing phaeohyphomycosis [102]. In our review, we found that antifungal therapy (48.4%) and antifungal therapy combined with surgery (35.7%) were the common choices for treating the infections caused by E. jeanselmei. The surgical resection was recommended for treating the phaeohyphomycosis in immunocompromised host, because the recurrence of the infection occurred in about 30.0% of these patients [107]. The surgical drainage was suggested when the surgical excision was not applicable for the patients [4]. Modifying immunosuppression condition could improve the outcome of antifungal treatment in transplant patients [6, 96, 108]. In terms of the patients’ outcome, we found that 76.2% of cases recovered. Poor outcome was usually associated with the misdiagnosis, invalid treatment, or the occurrence of disseminated infections [109].
We also found that more than 80.0% of the cases occurred in the patients over 50 years old and 95.2% of the related infections presented as localized subcutaneous or cutaneous lesions. The trauma, particularly in the face and extremity (61.9%), might be the common cause of the infections, which could lead to the inoculation of the fungus in the cutaneous or subcutaneous tissue as described previously [93, 110].
In addition, we payed attention to the correlation between immunity status and clinical characteristics of the infections caused by E. jeanselmei. As listed in Table 2, there were no statistically significant for the location of infection, treatment choice, length of treatment and patient’s outcome between immunocompromised groups and immunocompetent groups.
In all, the results of histopathological examination, culture and species identification can provide the important basis for precise diagnosis and effective treatment of the infection due to E. jeanselmei, as demonstrated in our case. The findings of our review based on the cases reported in PubMed database indicated that E. jeanselmei isolates had good in vitro susceptibility to antifungal agents and immune status of the patients might not be correlated to the characteristics of the infections.
References
Shields BE, Rosenbach M, Brown-Joel Z, Berger AP, Ford BA, Wanat KA. Angioinvasive fungal infections impacting the skin: background, epidemiology, and clinical presentation. J Am Acad Dermatol. 2019;80(4):869-880.e865.
Takenaka M, Murota H, Nishimoto K. Subcutaneous phaeohyphomycosis due to Exophiala jeanselmei following renal transplantation: a case report with a published work review of phaeohyphomycosis in Japan. J Dermatol. 2020;47(9):1050–3.
Bhardwaj S, Capoor MR, Kolte S, Purohit G, Dawson L, Gupta K, Ramesh V, Mandal AK. Phaeohyphomycosis due to Exophiala jeanselmei: an emerging pathogen in India—case report and review. Mycopathologia. 2016;181(3–4):279–84.
Lief MH, Caplivski D, Bottone EJ, Lerner S, Vidal C, Huprikar S. Exophiala jeanselmei infection in solid organ transplant recipients: report of two cases and review of the literature. Transp Infect Dis Off J Transpl Soc. 2011;13(1):73–9.
de Hoog GS, Vicente V, Caligiorne RB, Kantarcioglu S, Tintelnot K, van den Ende AHG, Haase G. Species diversity and polymorphism in the Exophiala spinifera clade containing opportunistic black yeast-like fungi. J Clin Microbiol. 2003;41(10):4767–78.
Puing AG, Couture-Cossette A, Wang AX, Zygourakis CC, Cheng X, Stevens BA, Banaei N, Novoa RA, Ho DY, Subramanian AK. Simultaneous coccidioidomycosis and phaeohyphomycosis in a kidney transplant recipient: a case report and literature review. Transpl Infect Dis Off J Transp Soc. 2020;22(6):e13365.
Wayne P. Clinical and laboratory standards institute (CLSI). reference method for broth dilution antifungal susceptibility testing of filamentous fungi, 3rd ed.CLSI document M38-A3. 2017.
Thammayya A, Sanyal M. Exophiala jeanselmei causing mycetoma pedis in India. Sabouraudia. 1980;18(2):91–5.
Monroe PW, Floyd WE. Chromohyphomycosis of the hand due to Exophiala jeanselmei (Phialophora jeanselmei, Phialophora gougerotii): case report and review. J Hand Surg. 1981;6(4):370–3.
Hironaga M, Mochizuki T, Watanabe S. Cutaneous phaeohyphomycosis of the sole caused by Exophiala jeanselmei and its susceptibility to amphotericin B, 5-FC and ketoconazole. Mycopathologia. 1982;79(2):101–4.
Prabhakar Y, Rao R, Sharma S, Bhatia VN, Arora AL, Srivastava KK, Yadav SS. A rare case of phaeohyphomycosis caused by Exophiala jeanselmei. Indian J Dermatol Venereol Leprol. 1983;49(1):17–21.
Zackheim HS, Halde C, Goodman RS, Marchasin S, Buncke HJ Jr. Phaeohyphomycotic cyst of the skin caused by Exophiala jeanselmei. J Am Acad Dermatol. 1985;12(1 Pt 2):207–12.
Sindhuphak W, MacDonald E, Head E, Hudson RD. Exophiala jeanselmei infection in a postrenal transplant patient. J Am Acad Dermatol. 1985;13(5 Pt 2):877–81.
Hemashettar BM, Patil CS, Nagalotimath SJ, Thammayya A. Mycetoma due to Exophiala jeanselmei (a case report with description of the fungus). Indian J Pathol Microbiol. 1986;29(1):75–8.
Padhye AA, Ajello L. A case of chromoblastomycosis with special reference to the mycology of the isolated Exophiala jeanselmei. Mykosen. 1987;30(3):134.
Hachisuka H, Matsumoto T, Kusuhara M, Nomura H, Nakano S, Sasai Y. Cutaneous phaeohyphomycosis caused by Exophiala jeanselmei after renal transplantation. Int J Dermatol. 1990;29(3):198–200.
Allred BJ. Subcutaneous phaeohyphomycosis due to Exophiala jeanselmei in an immunosuppressed patient: case report. N Zeal Med J. 1990;103(893):321–2.
Singh SM, Pouranik M, Naidu J. Cutaneous phaehyphomycosis caused by Exophiala jeanselmei var lecanii-cornii (Benedek and Specht) De Hoog. Indian J Pathol Microbiol. 1992;35(3):269–73.
Manian FA, Brischetto MJ. Pulmonary infection due to Exophiala jeanselmei: successful treatment with ketoconazole. Clin Infect Dis Off Publ Infect Dis Soc Am. 1993;16(3):445–6.
Agarwal S, Goodman NL, Malluche HH. Peritonitis due to Exophiala jeanselmei in a patient undergoing continuous ambulatory peritoneal dialysis. Am J Kidney Dis Off J Natl Kidney Found. 1993;21(6):673–5.
Schwinn A, Strohm S, Helgenberger M, Rank C, Bröcker EB. Phaeohyphomycosis caused by Exophiala jeanselmei treated with itraconazole. Mycoses. 1993;36(11–12):445–8.
De Hoog GS, Matsumoto T, Matsuda T, Uijthof JM. Exophiala jeanselmei var. lecanii-corni, an aetiologic agent of human phaeohyphomycosis, with report of a case. J Med Veterin Mycol Bi-Monthly Publ Int Soc Hum Animal Mycol. 1994;32(5):373–80.
Hayashi M, Kiryu H, Suenaga Y, Asahi M. A case of cutaneous infection by Exophiala jeanselmei. J Dermatol. 1994;21(12):971–3.
Kawachi Y, Tateishi T, Shojima K, Iwata M, Otsuka F. Subcutaneous pheomycotic cyst of the finger caused by Exophiala jeanselmei: association with a wooden splinter. Cutis. 1995;56(1):41–3.
Chuan MT, Wu MC. Subcutaneous phaeohyphomycosis caused by Exophiala jeanselmei: successful treatment with itraconazole. Int J Dermatol. 1995;34(8):563–6.
Whittle DI, Kominos S. Use of itraconazole for treating subcutaneous phaeohyphomycosis caused by Exophiala jeanselmei. Clin Infect Dis Off Publ Infect Dis Soc Am. 1995;21(4):1068.
Remon C, de la Calle IJ, Vallejo Carrion F, Perez-Ramos S, Fernández RE. Exophiala jeanselmei peritonities in a patient on CAPD. Periton Dial Int J Int Soc Periton Dial. 1996;16(5):536–8.
Kim HU, Kang SH, Matsumoto T. Subcutaneous phaeohyphomycosis caused by Exophiala jeanselmei in a patient with advanced tuberculosis. Br J Dermatol. 1998;138(2):351–3.
Xu X, Low DW, Palevsky HI, Elenitsas R. Subcutaneous phaeohyphomycotic cysts caused by Exophiala jeanselmei in a lung transplant patient. Dermatol Surg Off Publ Am Soc Dermatol Surg. 2001;27(4):343–6.
Aranegui B, Feal C, García CP, Batalla A, Abalde T, Alvarez-Martínez M, de la Torre C. Subcutaneous phaeohyphomycosis caused by Exophiala jeanselmei treated with wide surgical excision and posaconazole: case report. Int J Dermatol. 2013;52(2):255–6.
Boisseau-Garsaud AM, Desbois N, Guillermin ML, Ossondo M, Gueho E, Cales-Quist D. Onychomycosis due to Exophiala jeanselmei. Dermatol (Basel, Switzerl). 2002;204(2):150–2.
Liou JM, Wang JT, Wang MH, Wang SS, Hsueh PR. Phaeohyphomycosis caused by Exophiala species in immunocompromised hosts. J Formosan Med Assoc Taiwan yi zhi. 2002;101(7):523–6.
Murayama N, Takimoto R, Kawai M, Hiruma M, Takamori K, Nishimura K. A case of subcutaneous phaeohyphomycotic cyst due to Exophiala jeanselmei complicated with systemic lupus erythematosus. Mycoses. 2003;46(3–4):145–8.
Peña-Penabad C, Durán MT, Yebra MT, Rodríguez-Lozano J, Vieira V, Fonseca E. Chromomycosis due to Exophiala jeanselmei in a renal transplant recipient. Eur J Dermatol EJD. 2003;13(3):305–7.
Hague J, Liotta E. Subcutaneous phaeohyphomycosis caused by Exophiala jeanselmei in an immunocompromised host. Cutis. 2003;72(2):132–4.
Calista D, Leardini M, Arcangeli F. Subcutaneous Exophiala jeanselmei infection in a heart transplant patient. Eur J Dermatol EJD. 2003;13(5):489.
de Monbrison F, Piens MA, Ample B, Euvrard S, Cochat P, Picot S. Two cases of subcutaneous phaeohyphomycosis due to Exophiala jeanselmei, in cardiac transplant and renal transplant patients. Br J Dermatol. 2004;150(3):597–8.
Agger WA, Andes D, Burgess JW. Exophiala jeanselmei infection in a heart transplant recipient successfully treated with oral terbinafine. Clin Infect Dis Off Publ Infect Dis Soc Am. 2004;38(11):e112-115.
Silva Mdo R, Fernandes Ode F, Costa CR, Chaul A, Morgado LF, Fleury-Júnior LF, Costa MB. Subcutaneoous phaeohyphomycosis by Exophiala jeanselmei in a cardiac transplant recipient. Rev Inst Med Trop Sao Paulo. 2005;47(1):55–7.
Rallis E, Frangoulis E. Successful treatment of subcutaneous phaeohyphomycosis owing to Exophiala jeanselmei with oral terbinafine. Int J Dermatol. 2006;45(11):1369–70.
Arribi-Vilela A, Prats-Sánchez MD, Candel-González FJ, Andrade-Lobato R. Subcutaneous lesion in a renal transplant recipient. Enfermedades Infecciosas y Microbiol Clin. 2006;24(3):205–6.
Capoor MR, Khanna G, Nair D, Hasan A, Rajni DM, Aggarwal P. Eumycetoma pedis due to Exophiala jeanselmei. Indian J Med Microbiol. 2007;25(2):155–7.
Wakamatsu K, Takahata Y, Tokuhisa Y, Morita K, Muto M. Two cases of phaeohyphomycosis due to Exophiala jeanselmei. J Dermatol. 2008;35(3):178–80.
Martínez-González MC, Verea MM, Velasco D, Sacristán F, Del Pozo J, García-Silva J, Fonseca E. Three cases of cutaneous phaeohyphomycosis by Exophiala jeanselmei. Eur J Dermatol EJD. 2008;18(3):313–6.
Al-Tawfiq JA, Amr SS. Madura leg due to Exophiala jeanselmei successfully treated with surgery and itraconazole therapy. Med Mycol. 2009;47(6):648–52.
Umemoto N, Demitsu T, Kakurai M, Sasaki K, Azuma R, Iida E, Yoneda K, Kawasaki M, Mochizuki T. Two cases of cutaneous phaeohyphomycosis due to Exophiala jeanselmei: diagnostic significance of direct microscopical examination of the purulent discharge. Clin Exp Dermatol. 2009;34(7):e351-353.
Badali H, Najafzadeh MJ, van Esbroeck M, van den Enden E, Tarazooie B, Meis JF, de Hoog GS. The clinical spectrum of Exophiala jeanselmei, with a case report and in vitro antifungal susceptibility of the species. Med Mycol. 2010;48(2):318–27.
Arakaki O, Asato Y, Yagi N, Taira K, Yamamoto Y, Nonaka K, Hosokawa A, Kayo S, Hagiwara K, Uezato H. Phaeohyphomycosis caused by Exophiala jeanselmei in a patient with polymyalgia rheumatica. J Dermatol. 2010;37(4):367–73.
Rossetto AL, Dellatorre G, Pérsio RA, Romeiro JC, Cruz RC. Subcutaneous phaeohyphomycosis on the scrotum caused by Exophiala jeanselmei: case report. An Bras Dermatol. 2010;85(4):517–20.
Nomura M, Maeda M, Seishima M. Subcutaneous phaeohyphomycosis caused by Exophiala jeanselmei in collagen disease patient. J Dermatol. 2010;37(12):1046–50.
Parente JN, Talhari C, Ginter-Hanselmayer G, Schettini AP, Eiras Jda C, de Souza JV, Tavares R, Buzina W, Brunasso AM, Massone C. Subcutaneous phaeohyphomycosis in immunocompetent patients: two new cases caused by Exophiala jeanselmei and Cladophialophora carrionii. Mycoses. 2011;54(3):265–9.
Chen YC, Su YC, Tsai CC, Lai NS, Fan KS, Liu KC. Subcutaneous phaeohyphomycosis caused by Exophiala jeanselmei. J Microbiol Immunol Infect Wei Mian Yu Gan Ran Za zhi. 2014;47(6):546–9.
Zhou X, Hu Y, Hu Y, Liu K, Wang L, Wei Q, Han X, Zhu D, Lu Y, Mao Z, et al. Cutaneous and subcutaneous phaeohyphomycosis caused by Exophiala jeanselmei after renal transplantation: a case report. Nan fang yi ke da xue xue bao J Southern Med Univ. 2012;32(8):1206–10.
Fathy H, Abdel-Razek MM, Abdelgaber S, Othman T, El-Morsy F. Subcutaneous phaeohyphomycosis in immunocompetent child caused by Exophiala jeanselmei. Int J Dermatol. 2012;51(10):1267–70.
Pattanaprichakul P, Bunyaratavej S, Leeyaphan C, Sitthinamsuwan P, Sudhadham M, Muanprasart C, Feng P, Badali H, de Hoog GS. An unusual case of eumycetoma caused by Exophiala jeanselmei after a sea urchin injury. Mycoses. 2013;56(4):491–4.
Desoubeaux G, Millon A, Freychet B, de Muret A, Garcia-Hermoso D, Bailly E, Rosset P, Chandenier J, Bernard L. Eumycetoma of the foot caused by Exophiala jeanselmei in a Guinean woman. J Mycol Med. 2013;23(3):168–75.
Joshi P, Agarwal S, Singh G, Xess I, Bhowmik D. “A fine needle aspiration cytology in time saves nine”: cutaneous phaeohyphomycosis caused by Exophiala jeanselmei in a renal transplant patient: diagnosis by fine needle aspiration cytology. J Cytol. 2016;33(1):55–7.
Miyagawa F, Shobatake C, Fukumoto T, Yamanaka Y, Kobayashi N, Nishimura K, Masuda M, Asada H. Cutaneous phaeohyphomycosis caused by Exophiala jeanselmei in a healthy individual. J Dermatol. 2018;45(1):106–8.
Miyashita K, Matsuo A, Johno M, Noguchi H, Matsumoto T, Hiruma M, Kimura U, Kano R, Yaguchi T, Ihn H. Subcutaneous cystic phaeohyphomycosis caused by Exophiala jeanselmei. J Dermatol. 2019;46(12):e449–51.
Ramprasad A, Rastogi N, Xess I, Singh G, Ranjan P, Jadon R, Ray A, Vikram N. Disseminated phaeohyphomycosis by Exophiala jeanselmei. QJM Month J Assoc Phys. 2020;113(4):305.
Tirico MC, Neto CF, Cruz LL, Mendes-Sousa AF, Valkinir DE, Spina R, Oliveira WR. Clinical spectrum of phaeohyphomycosis in solid organ transplant recipients. JAAD Case Rep. 2016;2(6):465–9.
Ito A, Yamada N, Kimura R, Tanaka N, Kurai J, Anzawa K, Mochizuki T, Yamamoto O. Concurrent double fungal infections of the skin caused by phialemoniopsis endophytica and Exophiala jeanselmei in a patient with microscopic polyangiitis. Acta Dermato-Venereol. 2017;97(9):1142–4.
Leung EH, Moskalewicz R, Parada JP, Kovach KJ, Bouchard C. Exophiala jeanselmei keratitis after laser in situ keratomileusis. J Cataract Refract Surg. 2008;34(10):1809–11.
Hofling-Lima AL, Freitas D, Fischman O, Yu CZ, Roizenblatt R, Belfort R Jr. Exophiala jeanselmei causing late endophthalmitis after cataract surgery. Am J Ophthalmol. 1999;128(4):512–4.
Li DM, Li RY, de Hoog GS, Sudhadham M, Wang DL. Fatal Exophiala infections in China, with a report of seven cases. Mycoses. 2011;54(4):e136-142.
Chhonkar A, Kataria D, Tambe S, Nayak CS. Three rare cases of cutaneous phaeohyphomycosis. Indian J Plast Surg Off Publ Assoc Plast Surg India. 2016;49(2):271–4.
de Oliveira WR, Borsato MF, Dabronzo ML, Festa Neto C, Rocha LA, Nunes RS. Phaeohyphomycosis in renal transplantation: report of two cases. An Bras Dermatol. 2016;91(1):89–92.
Roncoroni AJ, Smayevsky J. Arthritis and endocarditis from Exophiala jeanselmei infection. Ann Intern Med. 1988;108(5):773.
da Silva Hellwig AH, Heidrich D, Zanette RA, Scroferneker ML. In vitro susceptibility of chromoblastomycosis agents to antifungal drugs: a systematic review. J Glob Antimicrob Resist. 2019;16:108–14.
Flynn BJ, Bourbeau PP, Cera PJ, Scicchitano LM, Jordan RL, Yap WT. Phaeohyphomycosis of the epididymis caused by Exophiala jeanselmei. J Urol. 1999;162(2):492–3.
Sartoris KE, Baillie GM, Tiernan R, Rajagopalan PR. Phaeohyphomycosis from Exphiala jeanselmei with concomitant Nocardia asteroides infection in a renal transplant recipient: case report and review of the literature. Pharmacotherapy. 1999;19(8):995–1001.
Nucci M, Akiti T, Barreiros G, Silveira F, Revankar SG, Sutton DA, Patterson TF. Nosocomial fungemia due to Exophiala jeanselmei var. jeanselmei and a Rhinocladiella species: newly described causes of bloodstream infection. J Clin Microbiol. 2001;39(2):514–8.
Iwatsu T, Miyaji M. Phaeomycotic cyst. A case with a lesion containing a wooden splinter. Arch Dermatol. 1984;120(9):1209–11.
Severo LC, Oliveira FM, Vettorato G, Londero AT. Mycetoma caused by Exophiala jeanselmei. Report of a case successfully treated with itraconazole and review of the literature. Rev Iberoam Micol. 1999;16(1):57–9.
Hammer ME, Harding S, Wynn P. Post-traumatic fungal endophthalmitis caused by Exophiala jeanselmei. Ann Ophthalmol. 1983;15(9):853–5.
Sautter RE, Bliss MD, Morrow D, Lee RE. Isolation of Exophiala jeanselmei associated with esophageal pathology: three cases, laboratory and clinical features. Mycopathologia. 1984;87(1–2):105–9.
Oberlin KE, Nichols AJ, Rosa R, Dejman A, Mattiazzi A, Guerra G, Elgart GW, Abbo LM. Phaeohyphomycosis due to Exophiala infections in solid organ transplant recipients: case report and literature review. Transpl Infect Dis Off J Transpl Soc. 2017, 19(4).
Hurtado I, Magran BL. Invasion of a soft contact lens by Exophiala jeanselmei. Mycopathologia. 1989;105(3):171–3.
Al-Hedaithy SS, Al-Kaff AS. Exophiala jeanselmei keratitis. Mycoses. 1993;36(3–4):97–100.
Pepe RR, Vigolo G. First isolation of Exophiala jeanselmei (Lang) De Hoog from a dental granuloma. Ann Osp Maria Vittoria Torino. 1986;29(7–12):283–91.
Saeedi OJ, Iyer SA, Mohiuddin AZ, Hogan RN. Exophiala jeanselmei keratitis: case report and review of literature. Eye Contact Lens. 2013;39(6):410–2.
Laverde S, Moncada LH, Restrepo A, Vera CL. Mycotic keratitis; 5 cases caused by unusual fungi. Sabouraudia. 1973;11(2):119–23.
Ben-Simon GJ, Barequet IS, Grinbaum A. More than tears in your eyes (Exophiala jeanselmei keratitis). Cornea. 2002;21(2):230–1.
Potel J, Rohde G, Seeliger H, Werry H. Exophiala jeanselmei (Phialophora jeanselmei): demonstration in the vitreous body of the eye. Mykosen. 1984;27(8):380–4.
Espinel-Ingroff A, Shadomy S, Kerkering TM, Shadomy HJ. Exoantigen test for differentiation of Exophiala jeanselmei and Wangiella dermatitidis isolates from other dematiaceous fungi. J Clin Microbiol. 1984;20(1):23–7.
Naka W, Harada T, Nishikawa T, Fukushiro R. A case of chromoblastomycosis with special reference to the mycology of the isolated Exophiala jeanselemei. Mycoses. 1988;31(2):70.
Komatsu-Fujii T, Nonoyama S, Ogawa M, Fukumoto T, Sakai C, Yoshimoto Y, Nakanishi K, Abe N, Tanabe H. Subcutaneous pseudocystic phaeohyphomycosis due to Exophiala jeanselmei mimicking an epidermal cyst. J Eur Acad Dermatol Venereol JEADV. 2020;34(11):e745–7.
Alves de Medeiros AK, Lodewick E, Bogaert DJ, Haerynck F, Van Daele S, Lambrecht B, Bosma S, Vanderdonckt L, Lortholary O, Migaud M, et al. Chronic and invasive fungal infections in a family with CARD9 deficiency. J Clin Immunol. 2016;36(3):204–9.
Galezowski A, Delyon J, Le Cleach L, Guégan S, Ducroux E, Alanio A, Lastennet D, Moguelet P, Dadban A, Leccia MT, et al. Deep cutaneous fungal infections in solid-organ transplant recipients. J Am Acad Dermatol. 2020;83(2):455–62.
Clancy CJ, Wingard JR, Hong NM. Subcutaneous phaeohyphomycosis in transplant recipients: review of the literature and demonstration of in vitro synergy between antifungal agents. Med Mycol. 2000;38(2):169–75.
Hoffmann Cde C, Danucalov IP, Purim KS, Queiroz-Telles F. Infections caused by dematiaceous fungi and their anatomoclinical correlations. An Bras Dermatol. 2011;86(1):138–41.
Khan SA. Calcaneal osteomyelitis caused by Exophiala jeanselmei in an immunocompetent child. J Bone Joint Surg Am. 2007;89(11):2547.
Seyedmousavi S, Netea MG, Mouton JW, Melchers WJ, Verweij PE, de Hoog GS. Black yeasts and their filamentous relatives: principles of pathogenesis and host defense. Clin Microbiol Rev. 2014;27(3):527–42.
Brandt ME, Warnock DW. Epidemiology, clinical manifestations, and therapy of infections caused by dematiaceous fungi. J Chemother (Florence, Italy). 2003;15(Suppl 2):36–47.
Valenzuela P, Legarraga P, Rabagliati R. Epidemiology of invasive fungal disease by filamentous fungi in the period 2005 to 2015, in a university hospital in Santiago, Chile. Revista Chilena de Infectologia Organo Oficial de la Sociedad Chilena de Infectologia. 2019;36(6):732–41.
Santos DW, Camargo LF, Gonçalves SS, Ogawa MM, Tomimori J, Enokihara MM, Medina-Pestana JO, Colombo AL. Melanized fungal infections in kidney transplant recipients: contributions to optimize clinical management. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2017;23(5):333.e339-333.e314.
McCarty TP, Baddley JW, Walsh TJ, Alexander BD, Kontoyiannis DP, Perl TM, Walker R, Patterson TF, Schuster MG, Lyon GM, et al. Phaeohyphomycosis in transplant recipients: results from the transplant associated infection surveillance network (TRANSNET). Med Mycol. 2015;53(5):440–6.
Revankar SG, Patterson JE, Sutton DA, Pullen R, Rinaldi MG. Disseminated phaeohyphomycosis: review of an emerging mycosis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2002;34(4):467–76.
Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev. 2010;23(4):884–928.
Wong EH, Revankar SG. Dematiaceous Molds. Infect Dis Clin N Am. 2016;30(1):165–78.
Silva WC, Gonçalves SS, Santos DW, Padovan AC, Bizerra FC, Melo AS. Species diversity, antifungal susceptibility and phenotypic and genotypic characterisation of Exophiala spp. infecting patients in different medical centres in Brazil. Mycoses. 2017;60(5):328–37.
Chowdhary A, Meis JF, Guarro J, de Hoog GS, Kathuria S, Arendrup MC, Arikan-Akdagli S, Akova M, Boekhout T, Caira M, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: diseases caused by black fungi. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2014;20(Suppl 3):47–75.
Zeng JS, Sutton DA, Fothergill AW, Rinaldi MG, Harrak MJ, de Hoog GS. Spectrum of clinically relevant Exophiala species in the United States. J Clin Microbiol. 2007;45(11):3713–20.
Arcobello JT, Revankar SG. Phaeohyphomycosis. Semin Resp Crit Care Med. 2020;41(1):131–40.
Yamazaki T, Inagaki Y, Fujii T, Ohwada J, Tsukazaki M, Umeda I, Kobayashi K, Shimma N, Page MG, Arisawa M. In vitro activity of isavuconazole against 140 reference fungal strains and 165 clinically isolated yeasts from Japan. Int J Antimicrob Agents. 2010;36(4):324–31.
Revankar SG, Nailor MD, Sobel JD. Use of terbinafine in rare and refractory mycoses. Fut Microbiol. 2008;3(1):9–17.
Abdolrasouli A, Gonzalo X, Jatan A, McArthur GJ, Francis N, Azadian BS, Borman AM, Johnson EM. Subcutaneous Phaeohyphomycosis Cyst Associated with Medicopsis romeroi in an Immunocompromised Host. Mycopathologia. 2016;181(9–10):717–21.
Aydın M, Özçelik Ü, Çevik H, Çınar Ö, Evren E, Demirağ A. Multiple brain abscesses due to phialemonium in a renal transplant recipient: first case report in the literature. Exp Clin Transpl Off J Middle East Soc Organ Transpl. 2015;13(Suppl 3):77–80.
Hsu CC, Chang SS, Lee PC, Chao SC. Cutaneous alternariosis in a renal transplant recipient: a case report and literature review. Asian J Surg. 2015;38(1):47–57.
Kantarcioglu AS, de Hoog GS. Infections of the central nervous system by melanized fungi: a review of cases presented between 1999 and 2004. Mycoses. 2004;47(1–2):4–13.
Najafzadeh MJ, Dolatabadi S, Vicente VA, de Hoog GS, Meis JF. In vitro activities of 8 antifungal drugs against 126 clinical and environmental Exophiala isolates. Mycoses. 2021;64(11):1328–33.
Singh S, Rudramurthy SM, Padhye AA, Hemashetter BM, Iyer R, Hallur V, Sharma A, Agnihotri S, Gupta S, Ghosh A, et al. Clinical spectrum, molecular characterization, antifungal susceptibility testing of Exophiala spp. from India and description of a novel Exophiala Species, E. arunalokei sp. nov. Front Cell Infect Microbiol. 2021;11:686120.
Borman AM, Fraser M, Szekely A, Larcombe DE, Johnson EM. Rapid identification of clinically relevant members of the genus Exophiala by matrix-assisted laser desorption ionization-time of flight mass spectrometry and description of two novel species, Exophiala campbellii and Exophiala lavatrina. J Clin Microbiol. 2017;55(4):1162–76.
Fothergill AW, Rinaldi MG, Sutton DA. Antifungal susceptibility testing of Exophiala spp.: a head-to-head comparison of amphotericin B, itraconazole, posaconazole and voriconazole. Med Mycol. 2009;47(1):41–3.
Odabasi Z, Paetznick VL, Rodriguez JR, Chen E, Ostrosky-Zeichner L. In vitro activity of anidulafungin against selected clinically important mold isolates. Antimicrob Agents Chemother. 2004;48(5):1912–5.
Acknowledgements
We thank the colleagues in clinical microbiology laboratory, West China Hospital of Sichuan University, for the suggestions of preparing the case report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statements
This work was already approved by the Ethics Committee of West China Hospital of Sichuan University (No. 954).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Abdullah Mohammed Said Al-Hatmi.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, C., Shu, L., Chen, Z. et al. Cutaneous Phaeohyphomycosis of the Right Hand Caused by Exophiala jeanselmei: A Case Report and Literature Review. Mycopathologia 187, 259–269 (2022). https://doi.org/10.1007/s11046-022-00623-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-022-00623-y