Abstract
Aspergillus endocarditis is a rare infection that may affect immunocompetent patients following heart valve replacement or heart surgery. We report the case of a 39 year old woman with a history of intravenous drug use who developed endocarditis with direct examination of the resected valve and vegetation showing the presence of mycelia. Cultures were positive for an Aspergillus of section Nigri, which was subsequently identified as Aspergillus tubingensis by sequencing. The clinical course was favorable following surgery and prolonged antifungal therapy (8 months in total). Antifungal susceptibility testing showed good in vitro activity of amphotericin B, voriconazole and echinocandins against planktonic cells of this A. tubingensis isolate. However, only amphotericin B displayed significant activity against biofilms. In vitro combinations of voriconazole or amphotericin B with echinocandins did not meet the criteria of synergism. Our review of the literature identified 17 other cases of endocarditis attributed to Aspergillus of section Nigri with an overall mortality rate of 57% (100% in the absence of surgery). Endocarditis caused by Aspergillus niger and related cryptic species are rare events, for which surgical management appears to be crucial for outcome. While amphotericin B was the only antifungal drug displaying significant anti-biofilm activity, the type and duration of antifungal therapy remain to be determined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Aspergillus spp. are opportunistic mold pathogens causing invasive aspergillosis in patients with severe immunosuppression, such as hematologic cancer patients or transplant recipients [1]. While the lung represents the main port of entry, primary extra-pulmonary infections are occasionally observed. Aspergillus endocarditis is a very rare entity, which has been reported not only in immunocompromised patients, but also in immunocompetent individuals with a history of valve replacement, open heart surgery, or intravenous drug use [2,3,4]. In this setting, direct inoculation of the fungus in blood may occur via contaminated material. Aspergillus endocarditis is notoriously difficult to treat in the absence of evidence-based recommendations regarding the type and duration of antifungal therapy [3]. Valve replacement is considered to be mandatory [3]. The mortality rate is very high and there is an important risk of relapsing infection among survivors, which usually poses the indication for prolonged suppressive therapy [3].
While Aspergillus fumigatus represents the major cause of Aspergillus endocarditis and invasive aspergillosis in general, Aspergillus niger and other Aspergillus spp. of section Nigri account for some cases [5,6,7,8,9,10,11,12,13,14,15,16,17,18]. Indeed, Aspergillus niger is ubiquitous in the environment and has been recognized as a relatively frequent contaminant of hospital indoor environment (e.g. following building renovation works) or infusion fluids (e.g. peritoneal dialysate, dextrose infusion) [19,20,21,22]. Among Aspergillus species of section Nigri, cryptic species are frequently recovered in clinical specimens with Aspergillus tubingensis accounting for about 20% to over 50% of them [23,24,25,26,27]. This pathogen has been associated with decreased azole susceptibility in some cases [24,25,26].
We report here a case of A. tubingensis prosthetic valve endocarditis in an apparently immunocompetent patient known for intravenous drug use, which was successfully treated with surgery and combined antifungal therapy. This work was completed by a review of the literature of Aspergillus section Nigri endocarditis and by in vitro experiments of anti-planktonic and anti-biofilm activity of the different antifungal drugs against the isolated pathogen.
Case Presentation
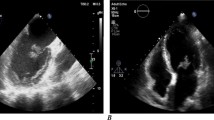
A 39 year-old woman known for active intravenous drug use (mainly cocaine) and a biological aortic valve replacement for Enterococcus faecalis endocarditis more than 10 years ago was admitted to the emergency room for altered level of consciousness and septic shock. Blood cultures drawn at admission were positive for Proteus mirabilis (all four bottles from two distinct pairs). Her hemodynamic condition rapidly improved after initiation of broad-spectrum antibiotic therapy and subsequent switch for ceftriaxone and addition of gentamicin after identification of the bacterial blood pathogen. A transoesphageal cardiac ultrasound revealed an 11 mm motile element on the right coronary leaflet of the aortic valve (Fig. 1, panel A). The patient underwent biological replacement of the aortic valve. A piece of the resected valvular tissue and vegetation was sent to the laboratory of microbiology. Direct examination did not reveal the presence of bacteria at gram staining, but thin septate and branched mycelial elements consistent with an Aspergillus spp. were visualized at silver staining (Fig. 1, panel B). Conventional cultures of the resected valve did not reveal the presence of bacteria, but a filamentous fungus grew on all plates after 24 h of incubation, which was subsequently identified as an Aspergillus of section Nigri. The direct panfungal PCR (targeting the 18S rDNA) was positive for an Aspergillus of section Nigri. Of note, the direct eubacterial PCR (targeting the 16S rDNA) was also positive for P. mirabilis. Another piece of the cardiac valve was also sent for histopathological examination, which showed fibrino-leucocytic debris without mycelial elements at Grocott staining.
Case report: radiological and microbiological images. Panel A Image of the transoesophageal cardiac echography showing a motile element of 11 mm (yellow arrow) on the right coronary leaflet of the biological prosthetic aortic valve. Panel B Image of the direct examination of the valvular tissue after silver staining showing mycelial elements with septate and 45° branching hyphae consistent with an Aspergillus spp
Antifungal susceptibility testing of the Aspergillus section Nigri was performed by Sensititre YeastOne™ (Trek Diagnostics Systems, ThermoFisher Scientific, Cleveland, OH, USA) according to manufacturer’s recommendations. Minimal inhibitory concentrations (MIC) were as follow: amphotericin B 1 mg/l, voriconazole 1 mg/l, itraconazole 1 mg/l, posaconazole 0.25 mg/l, caspofungin 0.015 mg/l, anidulafungin < 0.015 mg/l, micafungin < 0.008 mg/l. Although interpretive clinical breakpoints are lacking for Aspergillus of section Nigri, these values were below the reported epidemiological cut-off values (ECVs) and were considered as indicative to guide antifungal therapy [28,29,30].
The galactomannan and 1,3-beta-d-glucan were positive in serum (optical density 2.34 and > 500 pg/ml, respectively). The diagnostic work-up was completed by a cerebral and thoraco-abdominal CT-scan that did not show evidence of secondary foci of the infection. A diagnosis of mixed bacterial (P. mirabilis) and fungal (A. niger) endocarditis secondary to intravenous drug injection was established. Intravenous liposomal amphotericin B (L-AMB, 5 mg/kg/day) was started with addition of caspofungin (70 mg loading dose, followed by 50 mg/day from day 2). Three days later, L-AMB was substituted for intravenous voriconazole (6 mg/kg bid on day 1, followed by 4 mg/kg bid from day 2) and gentamicin was interrupted due to the development of acute renal failure. The clinical and biological course was favorable with serum galactomannan rapidly turning negative and a decline of serum 1,3-beta-d-glucan. Documentation of the clearance of the P. mirabilis bacteremia was obtained at day 5 from the start of antibacterial therapy and the patient was treated with ceftriaxone for a total of 6 weeks after surgery. The patient was discharged at post-operative day 10 and was followed at the outpatient clinic for monthly clinical evaluation and laboratory tests (hepatic tests, creatinine, complete blood count). Voriconazole was switched from intravenous to oral administration after 7 days and combination therapy of voriconazole and caspofungin was continued for a total of 14 days. The trough concentration of voriconazole measured at that time (day 14) was 4.5 mg/l. Voriconazole monotherapy was continued for a total of 47 days and was then substituted for oral isavuconazole (200 mg tid on days 1 and 2, followed by 200 mg qd) because of abnormal hepatic tests. The PET-CT performed 6 months after surgery showed no signs of recurrent infection. Isavuconazole was stopped after a total of 8 months of antifungal therapy because of shortage of the drug and multiple interactions with the patient’s psychotropic medications (risperidone, pregabalin, clorazepate, trazodone) precluding administration of other azole drugs. About 6 months later, the patient developed a novel episode of endocarditis, which was suspected to be recurrent Aspergillus endocarditis, despite a negative galactomannan in serum. However, microbiological analyses of the resected valve established the diagnosis of Saccharomyces cerevisiae endocarditis without any evidence of recurrent Aspergillus infection [31].
Methods
The mold recovered by culture of the infected cardiac valve and identified as an Aspergillus of section Nigri by morphological examination was further characterized at the species level by beta-tubulin (BenA) sequencing using primers previously described [32]. Drug interactions were tested by the checkerboard dilution method using the broth microdilution protocol of the Clinical and Laboratory Standards Institute (CLSI) M38 (3rd edition) for MIC determination [33]. The fractional inhibitory concentration index (FICI) was calculated and interpreted as previously described [34]. In order to test the antifungal activity against the Aspergillus biofilms, we measured the reduction of the tetrazolium salt 2,3-bis(2-methoxy-4-nitro-5-[(sulphenylamino)carbonyl]-2H-tetrazolium-hydroxide (XTT) by metabolic active fungal cells within the biofilm, as previously described [35, 36]. In brief, from a suspension of 2.103 conidia/mL in MOPS-buffered RPMI 1640 (Sigma-Aldrich, Saint-Louis, MO), 200 µL were incubated for 48 h at 37 °C in a 96-well plate (Corning, NY, USA). Biofilms were washed with PBS twice to eliminate non-adherent cells and incubated at 37 °C for 16 h in MOPS-buffered RPMI 1640 with addition of antifungals (amphotericin B, voriconazole or caspofungin, Sigma-Aldrich, Saint-Louis, MO) at different concentrations. Wells were rinsed with PBS twice to eliminate antifungals and incubated with 200 µL of a saline solution containing 200 µg/mL XTT and 25 µM menadione (Sigma-Aldrich, Saint-Louis, MO) for 2 h at 37 °C in the dark. The color change was measured by the LUMIstarOmega microplate reader (BMG LABTECH, Ortenberg, Germany) using a 485 nm filter. The sessile minimal inhibitory concentration (SMIC) was assessed as the concentration of the drug achieving a 50% decrease of absorbance.
Results
The pathogenic mold was identified as Aspergillus tubingensis by sequencing of BenA showing a total scores of 898 (100% similitude with A. tubingensis voucher IHEM17170 BenA gene) and 929 (99% similitude with A. tubingensis isolate A87CM CaM gene). These sequences as been deposited in GenBank under ID numbers OL771245. In checkerboard dilutions, the interactions of amphotericin B/caspofungin and voriconazole/caspofungin were classified as indifferent with FICIs of 0.75 and 1.125, respectively. Results of the XTT assay are shown in Fig. 2. Only amphotericin B displayed significant activity against sessile cells with a SMIC of 1 µg/ml. Addition of caspofungin to either amphotericin B or voriconazole did not result in enhanced activity.
Anti-biofilm activity of antifungal drugs against Aspergillus tubingensis. The anti-biofilm activity of antifungal drugs was measured against the sessile forms of the A. tubingensis isolate of the present case using the XTT reduction assay. Absorbance (λ485nm) was measured after 16 h of drug exposure and expressed as relative optical density (OD). A Amphotericin B (AmB) alone and combined with caspofungin (CAS, fixed dose of 0.25 µg/ml), B voriconazole (VRC) alone and combined with caspofungin (CAS, fixed dose of 0.25 µg/ml), C caspofungin, D isavuconazole. The sessile minimal inhibitory concentration (SMIC), defined as the concentration of the drug achieving a 50% decrease of relative OD, was 1 µg/ml, > 4 µg/ml, > 1 µg/ml, and > 8 µg/ml for amphotericin B, voriconazole, caspofungin and isavuconazole, respectively. The addition of caspofungin to amphotericin B or voriconazole did not result in any additive effect
Review of the Literature
A systematic search was performed on PubMed (https://pubmed.ncbi.nlm.nih.gov/) using the terms: “endocarditis” or “aortitis” and “Aspergillus niger” or “Aspergillus tubingensis” or “section Nigri”. Our search identified 17 cases of endocarditis or aortitis caused by Aspergillus spp. of section Nigri. Description of these cases with addition of the present case report (n = 18) is provided in Table 1. All patients except one had a past history of heart surgery and/or heart valve replacement or valvuloplasty. Only one patient was immunocompromised (acute leukemia). Septic embolisms were commonly observed. Mortality was 100% in the absence or surgical intervention and 45% among patients who underwent surgery of valve replacement and/or thrombus removal. Among the patients who survived without recurrence in follow-up, the duration of antifungal therapy ranged from 6 to 12 months.
Discussion and Conclusions
We reported the case of a patient who developed A. tubingensis endocarditis on a biological prosthetic valve, probably following direct inoculation of the pathogen in the blood via a contaminated drug injection, in the absence of other primary focus of infection. The patient was successfully treated by surgery and 8 months of antifungal therapy consisting of initial L-AMB therapy for 3 days followed by a combination of voriconazole and caspofungin for 14 days and then voriconazole and isavuconazole monotherapy.
Aspergillus endocarditis is a rare event and represents the second cause of fungal endocarditis after Candida spp. [37, 38]. Prosthetic heart valves, structural heart disease or past history of endocarditis were the most frequent underlying conditions [37, 38]. History of active intravenous drug abuse has also been reported [37, 39], although it is unclear whether the fungus originates from the drug or its adjuvant, or from the contaminated material of injection.
Our review of the literature identified 17 case reports of endocarditis caused by Aspergillus spp. of section Nigri. Most of these cases were attributed to A. niger by phenotypic identification, but were not characterized at the species level by molecular analysis. Our case represents the first report of endocarditis attributed to A. tubingensis, which is actually one of the most frequent cryptic species of section Nigri in Europe [24, 25, 27]. This species may exhibit some decrease of azole susceptibility compared to A. niger sensu stricto, according to previous in vitro studies [24,25,26]. Our strain had a voriconazole MIC of 1 mg/l, which actually does not exceed the epidemiological cut-off value (i.e. 2 mg/l) of A. niger [29]. Data about the in vitro interactions of voriconazole with caspofungin (or other echinocandins) against Aspergillus spp. are controversial and suggest an occasional synergistic effect, which may be strain-dependent [40,41,42]. Data for A. niger are scarce, but the interaction was classified as indifferent for a majority of isolates [41]. Similar observations have been reported for the interaction of amphotericin B and caspofungin against Aspergillus spp. and A. niger [43]. Our checkerboard testing with the present A. tubingensis strain showing indifferent interactions for these antifungal combinations are consistent with these previous observations. Data on antifungal activity on biofilms are more scarce. One study assessed the individual and combined effect of voriconazole, amphotericin B and caspofungin against 22 Aspergillus spp. including 3. A. niger [44]. Our results are comparable to their observations with only amphotericin B displaying significant in vitro activity against the biofilm of A. tubingensis (SMIC 1 mg/l). However, contrarily to the results of Liu et al. [44], we did not observe any enhanced activity with the addition of caspofungin at therapeutic concentrations to either amphotericin B or voriconazole.
While the optimal antifungal therapy of Aspergillus endocarditis remains to be determined, our review of the literature shows that surgery is a key determinant of therapeutic success, which is in accordance with experts’ recommendations [3, 4]. The crucial role of surgery is not surprising as secondary embolic events represent a very frequent complication and the major cause of death among these patients. Fortunately, our patient did not experience any symptom or radiological evidence of thrombo-embolic complications before surgery, which was probably a good prognostic factor.
Another debated question in the management of Aspergillus endocarditis is the duration of antifungal therapy. In our literature review, all patients who survived without recurrence received antifungal therapy for at least 6 months. Whether this duration can be shortened is unknown. The use of PET-CT in follow-up may help in determining the duration of treatment as it was the case here.
In conclusion, this case of A. tubingensis endocarditis is the first one formally attributed to this cryptic species and among the rare ones attributed to Aspergillus of section Nigri that have been reported until now. Our in vitro analyses suggest that amphotericin B is the only antifungal drug displaying significant anti-biofilm activity, which may support its use as first-line therapy. We did not observe any benefit of the addition of an echinocandin on the basis of our in vitro data for the present case, but clinical data are lacking to determine the optimal antifungal regimen for such rare infections.
Data Availability
The dataset of this case report is available upon reasonable request of the editors and with respect of the confidential rules of our institution and anonymity of the patient.
Code Availability
Not applicable.
References
Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan SP, Meier-Kriesche HU, et al. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J Infect. 2012;65(5):453–64.
El-Hamamsy I, Durrleman N, Stevens LM, Perrault LP, Carrier M. Aspergillus endocarditis after cardiac surgery. Ann Thorac Surg. 2005;80(1):359–64.
Pasha AK, Lee JZ, Low SW, Desai H, Lee KS, Al MM. Fungal endocarditis: update on diagnosis and management. Am J Med. 2016;129(10):1037–43.
Tattevin P, Revest M, Lefort A, Michelet C, Lortholary O. Fungal endocarditis: current challenges. Int J Antimicrob Agents. 2014;44(4):290–4.
Arnaiz-Garcia ME, Gonzalez-Santos JM, Amoros-Rivera C, Arevalo-Abascal A. Aspergillus niger prosthetic valve endocarditis. Eur J Cardiothorac Surg. 2019;55(5):1018.
Badiee P, Alborzi A, Shakiba E, Ziyaeyan M, Pourabbas B. Molecular diagnosis of Aspergillus endocarditis after cardiac surgery. J Med Microbiol. 2009;58(Pt 2):192–5.
Badiee P, Amirghofran AA, Ghazi NM. Evaluation of noninvasive methods for the diagnosis of fungal endocarditis. Med Mycol. 2014;52(5):530–6.
Duygu H, Nalbantgil S, Ozerkan F, Kirilmaz B, Yagdi T. Aspergillus niger aortitis after aortic valve replacement diagnosed by transesophageal echocardiography. Echocardiography. 2006;23(5):405–6.
El-Hamamsy I, Durrleman N, Stevens LM, Cartier R, Pellerin M, Perrault LP, et al. A cluster of cases of Aspergillus endocarditis after cardiac surgery. Ann Thorac Surg. 2004;77(6):2184–6.
Jamieson RW, Wallace WA, Din JN, Raza Z. Acute aortic occlusion with sudden paraplegia secondary to Aspergillus niger embolism from Aspergillus niger aortitis. J Vasc Surg. 2011;54(5):1472–4.
Kocazeybek B, Sonmez B, Sarman K, Sener D, Ozdemirli M, Aytekin S, et al. Diagnosis at first glance: hairy black colonies in resected prosthetic aortic valve. Clin Microbiol Infect. 2000;6(11):617–8.
Kreiss Y, Vered Z, Keller N, Kochva I, Sidi Y, Gur H. Aspergillus niger endocarditis in an immunocompetent patient: an unusual course. Postgrad Med J. 2000;76(892):105–6.
Malivi TA, Webb HM, Dixon CD, Boone JA. Systemic aspergillosis caused by Aspergillus niger after open-heart surgery. JAMA. 1968;203(7):520–2.
Marro M, Atzeni F, La Torre MW, Attisani M, Belloro S, De Rosa FG, et al. Insidious postoperative Aspergillus niger graft aortitis. IDCases. 2020;21:e00823.
McCracken D, Barnes R, Poynton C, White PL, Isik N, Cook D. Polymerase chain reaction aids in the diagnosis of an unusual case of Aspergillus niger endocarditis in a patient with acute myeloid leukaemia. J Infect. 2003;47(4):344–7.
Moore RS, Hasleton PS, Lawson RA, Stanbridge TN. Aspergillus niger endocarditis complicating aortic tissue valve replacement. Thorax. 1984;39(1):76–7.
Noordally SO, Sohawon S, De Bels D, Duttmann R, Gottignies P, Devriendt J. Late onset of Aspergillus aortitis presenting as femoral artery embolism following coronary artery bypass graft surgery. Acta Med (Hradec Kralove). 2011;54(4):175–6.
Vivas C. Endocarditis caused by Aspergillus niger: case report. Clin Infect Dis. 1998;27(5):1322–3.
Dotis J, Kondou A, Koukloumperi E, Karava V, Papadopoulou A, Gkogka C, et al. Aspergillus peritonitis in peritoneal dialysis patients: a systematic review. J Mycol Med. 2020;30(4):101037.
Gupta A, Gupta V, Dogra MR, Chakrabarti A, Ray P, Ram J, et al. Fungal endophthalmitis after a single intravenous administration of presumably contaminated dextrose infusion fluid. Retina. 2000;20(3):262–8.
Laurel VL, Meier PA, Astorga A, Dolan D, Brockett R, Rinaldi MG. Pseudoepidemic of Aspergillus niger infections traced to specimen contamination in the microbiology laboratory. J Clin Microbiol. 1999;37(5):1612–6.
Loudon KW, Coke AP, Burnie JP, Shaw AJ, Oppenheim BA, Morris CQ. Kitchens as a source of Aspergillus niger infection. J Hosp Infect. 1996;32(3):191–8.
Balajee SA, Kano R, Baddley JW, Moser SA, Marr KA, Alexander BD, et al. Molecular identification of Aspergillus species collected for the transplant-associated infection surveillance network. J Clin Microbiol. 2009;47(10):3138–41.
Carrara B, Richards R, Imbert S, Morio F, Sasso M, Zahr N, et al. Species distribution and comparison between EUCAST and gradient concentration strips methods for antifungal susceptibility testing of 112 Aspergillus section Nigri isolates. Antimicrob Agents Chemother. 2020. https://doi.org/10.1128/AAC.02510-19.
Howard SJ, Harrison E, Bowyer P, Varga J, Denning DW. Cryptic species and azole resistance in the Aspergillus niger complex. Antimicrob Agents Chemother. 2011;55(10):4802–9.
Li Y, Wan Z, Liu W, Li R. Identification and susceptibility of Aspergillus section Nigri in China: prevalence of species and paradoxical growth in response to echinocandins. J Clin Microbiol. 2015;53(2):702–5.
Vermeulen E, Maertens J, Meersseman P, Saegeman V, Dupont L, Lagrou K. Invasive Aspergillus niger complex infections in a Belgian tertiary care hospital. Clin Microbiol Infect. 2014;20(5):O333–5.
Espinel-Ingroff A, Cuenca-Estrella M, Fothergill A, Fuller J, Ghannoum M, Johnson E, et al. Wild-type MIC distributions and epidemiological cutoff values for amphotericin B and Aspergillus spp. for the CLSI broth microdilution method (M38–A2 document). Antimicrob Agents Chemother. 2011;55(11):5150–4.
Espinel-Ingroff A, Diekema DJ, Fothergill A, Johnson E, Pelaez T, Pfaller MA, et al. Wild-type MIC distributions and epidemiological cutoff values for the triazoles and six Aspergillus spp. for the CLSI broth microdilution method (M38–A2 document). J Clin Microbiol. 2010;48(9):3251–7.
Pfaller MA, Boyken L, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, et al. In vitro susceptibility of clinical isolates of Aspergillus spp. to anidulafungin, caspofungin, and micafungin: a head-to-head comparison using the CLSI M38–A2 broth microdilution method. J Clin Microbiol. 2009;47(10):3323–5.
Tozzi P, Kampouri EE, Tzimas G, Prior JO, Monney P, Kamani C, et al. COVID-19 pandemics: a surprising link to bread flour with collateral damage to a prosthetic heart valve. Circ Cardiovasc Imaging. 2020;13(10):e011395.
Samson RA, Visagie CM, Houbraken J, Hong SB, Hubka V, Klaassen CH, et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud Mycol. 2014;78:141–73.
Clinical and Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: second edition (M38–A3). Wayne: Clinical and Laboratory Standards Institute; 2017.
Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52(1):1.
Meletiadis J, Mouton JW, Meis JF, Bouman BA, Donnelly JP, Verweij PE, et al. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J Clin Microbiol. 2001;39(9):3402–8.
Pierce CG, Uppuluri P, Tristan AR, Wormley FL Jr, Mowat E, Ramage G, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3(9):1494–500.
Meena DS, Kumar D, Agarwal M, Bohra GK, Choudhary R, Samantaray S, et al. Clinical features, diagnosis and treatment outcome of fungal endocarditis: a systematic review of reported cases. Mycoses. 2021;65(3):294–302.
Pierrotti LC, Baddour LM. Fungal endocarditis, 1995–2000. Chest. 2002;122(1):302–10.
Petrosillo N, Pellicelli AM, Cicalini S, Conte A, Goletti D, Palmieri F. Endocarditis caused by Aspergillus species in injection drug users. Clin Infect Dis. 2001;33(8):e97–9.
Manavathu EK, Alangaden GJ, Chandrasekar PH. Differential activity of triazoles in two-drug combinations with the echinocandin caspofungin against Aspergillus fumigatus. J Antimicrob Chemother. 2003;51(6):1423–5.
Perea S, Gonzalez G, Fothergill AW, Kirkpatrick WR, Rinaldi MG, Patterson TF. In vitro interaction of caspofungin acetate with voriconazole against clinical isolates of Aspergillus spp. Antimicrob Agents Chemother. 2002;46(9):3039–41.
Planche V, Ducroz S, Alanio A, Bougnoux ME, Lortholary O, Dannaoui E. In vitro combination of anidulafungin and voriconazole against intrinsically azole-susceptible and -resistant Aspergillus spp. Antimicrob Agents Chemother. 2012;56(8):4500–3.
Arikan S, Lozano-Chiu M, Paetznick V, Rex JH. In vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrob Agents Chemother. 2002;46(1):245–7.
Liu W, Li L, Sun Y, Chen W, Wan Z, Li R, et al. Interaction of the echinocandin caspofungin with amphotericin B or voriconazole against Aspergillus biofilms in vitro. Antimicrob Agents Chemother. 2012;56(12):6414–6.
Acknowledgements
We are grateful to the patient for her consent for this publication and to the medical staff involved in the management of this patient.
Funding
Open access funding provided by University of Lausanne. This work was done as part of our routine practice without any specific funding.
Author information
Authors and Affiliations
Contributions
TB: data collection, review of literature, in vitro experiments, drafting of manuscript. MA: in vitro experiments, figure design, review of manuscript. EK: data collection, review of literature, review of manuscript. MM: data collection, review of manuscript. PM: data collection, figure design, review of manuscript. PT: data collection, review of manuscript. FL: study design, data collection, review of literature, writing of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
F. Lamoth has received research funding from the Swiss National Science Foundation, the Santos-Suarez Foundation, Novartis, MSD and Pfizer outside of the scope of the present work, and has contributed to advisory boards for Gilead.
Ethical Approval
Not applicable, according to the local ethics requirements about case reports.
Consent to Participate
Not applicable.
Consent for Publication
Written informed consent was obtained from the patient for the publication of this case report.
Additional information
Handling Editor: Ruoyu Li.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Born, T., Aruanno, M., Kampouri, E. et al. Aspergillus tubingensis Endocarditis: A Case Report and Review of the Literature. Mycopathologia 187, 249–258 (2022). https://doi.org/10.1007/s11046-022-00621-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-022-00621-0