Abstract
The prevalence of black fungi in the order Chaetothyriales has often been underestimated due to the difficulty of their isolation. In this study, three methods which are often used to isolate black fungi are compared. Enrichment on aromatic hydrocarbon appears effective in inhibiting growth of cosmopolitan microbial species and allows appearance of black fungi. We miniaturized the method for high-throughput purposes. The new procedure saves time, consumes less space and can process multiple samples simultaneously.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Black yeasts in the order Chaetothyriales are renowned for their polyextremotolerance, i.e., survival under conditions of osmotic, nutrient and toxin stress [1]. The derived family Herpotrichiellaceae also contains a large number of species potentially causing infections in humans [2, 3]. Their prevalence has been underestimated due to the difficulty of their isolation in culture, caused by slow growth, low competitive ability and oligotrophic nature as a result of which they prevalently occupy (micro)habitats that are hostile to microbial life [4]. Cell walls in all species are invariably melanized to enhance protection against solar irradiation and dryness, while many species possess pathways for assimilation of monoaromatic alkylbenzenes [5]. Polyextremotolerance enables members of Chaetothyriales to reside in highly diverse habitats and under a wide range of environmental conditions. A remarkably large share of the known species are opportunistic pathogens on mammals including humans and amphibians [6, 7], sometimes causing disseminated or neurological, potentially fatal disorders. The route of infection for most of these fungi is unknown, and therefore, environmental screening and isolation are essential to enable preventive methods in public health programs.
If the problematic isolation of chaetothyrialean black yeasts is due to more rapidly growing saprobic competitors in the samples, methods of selectivity and enrichment should be developed. In most common habitats, conventional culturing is not effective [8]. An oil flotation isolation technique has successfully been applied to isolate black fungi and relatives from the environment [9]. This technique applies mineral oil to selectively recover black yeast conidia. Zhao et al. [10] applied an enrichment technique proposed by Prenafeta-Boldú et al. [11] based on solid-state-like incubations in a controlled atmosphere containing monoaromatic volatile hydrocarbons, usually toluene, as sole carbon source [10, 11]. Perlite granule is a very suitable support for fungal growth (inert material, highly microporous with the good water holding capacity of 65%, and can provide the high contrast for visualization of 66 melanized fungal strains due to its white color). Besides the volatile substrates for growth, pH and water activity can also be adjusted for a selective enrichment of fungi. The method provided positive results from samples of natural environments as well as hydrocarbon-polluted habitats [8, 10, 12]. The disadvantage of this method is that it is slow and requires rather large containers (serum flasks enclosed in glass desiccators to prevent the leakage of volatile substrates) such that the number of samples that can be processed per batch is limited. Our aim is to develop a high-throughput methodology based on the solid-state-like enrichment on volatile monoaromatic hydrocarbons for the isolation of black fungi. We compared the effectivity of conventional culturing, oil flotation and miniaturized enrichment for the isolation of two model strains from sterilized compost and from raw compost which are rich in microbial competitors.
Materials and Methods
Strains and Samples
Exophiala dermatitidis CBS 207.35 was grown for 7 days on malt extract agar (MEA, Oxoid) medium. Fresh transfers were made on MEA on slants for 7 days at 28 °C. Suspensions were made in sterilized physiological salt solution with a sterile cotton swab, and cells were counted using a Neubauer’s chamber. Cell densities were adjusted to 1 × 106 CFU/mL. An environmental strain, Exophiala xenobiotica N1 from an oak railway sleeper in Nijmegen, the Netherlands, was used according to the same protocol.
About 1 g of sterilized compost (Florentus, Zuidwolde, the Netherlands) was transferred to test tubes containing 9 mL sterilized water. Samples were homogenized for 1 min in a MoBio vortex and used as suspension. One mL aliquots of the samples were spiked separately with 1 mL Exophiala CBS 207.35 suspension and within tubes containing 8 mL sterilized water. Cell densities of black yeasts in these tubes were 1 × 105. One gram of raw compost was treated according to the same protocol. Aspergillus fumigatus V139-36 and Fusarium sp. V179-73 were used for competition tests in dilutions of 106–102 CFU/mL.
Isolation Protocols
Method 1: Conventional Culturing Compost and spiked suspensions were processed as a dilution series: (units: CFU/mL) 1 × 105, 1 × 104, 1 × 103, 1 × 102. About 0.1 mL of three concentrations (1 × 104, 1 × 103 and 1 × 102) was pipetted onto a 2% MEA plate containing antibiotics (200 U penicillin and 200 µg/L streptomycin). Plates were incubated at 28 °C for 7 days, and black yeast colonies were counted.
Method 2: Isolation by Oil Flotation Methods applied were those of Satow et al. [13]. One mL aliquots of each sample were added to Erlenmeyer flasks containing 100 mL sterile salt solution (0.9% NaCl, 200 U penicillin, 200 µg/L streptomycin, 200 µg/L chloramphenicol, 500 µg/L cycloheximide). Samples were incubated for 30 min at room temperature in standstill. Subsequently, 20 mL sterile mineral oil was dispensed into the flasks and the solutions were vigorously shaken for 5 min. After 20 min, the interface was collected, formulated into series concentration suspensions, pipetted onto MEA plates and incubated at 28 °C for 4 weeks.
Method 3 (Fig. 1): Isolation by Solid-State-Like Enrichment Sealed jars of 200 mL were sterilized and filled with approximately 4 g of perlite granules saturated with 50 mL mineral medium [14]. Forty-nine mL saline phosphate buffer (8 g/L NaCl, 0.2 g/L KCl, 1.78 g/L Na2HPO4, 0.27 g/L KH2PO4) and 1 mL sample solution were inoculated into the jars. A small open glass vial was placed in the middle of the jar. A toluene gas phase was generated by adding 10 mL of a 5% (v/v) solution of the aromatic substrate in dibutyl phthalate to the glass vial. Jars were sealed airtight with plastic bag matching cover and incubated at room temperature for 2 months. After opening the jars, 0.1 mL aliquots of 104, 103 and 102 dilutions of each sample were inoculated in duplicate on 2% MEA plates containing penicillin and streptomycin and incubated at 28 °C. Fungal growth was observed daily, and black yeast-like and contaminating colonies were counted. This part of the experiment is carried out in a safety cabinet to avoid the contamination of toluene.
Competition
Competitive ability was tested with two fungi commonly occurring in compost (Aspergillus fumigatus V139-36 and Fusarium sp. V179-73) selected to represent fast-growing fungi. Conidial suspensions (106–102) of Exophiala dermatitidis CBS 207.35 and of the selected contaminants were made by a dilution series (106–102 CFU/mL). Using conventional culturing, at each concentration, CBS 207.35 was inoculated together on agar plates with A. fumigatus and Fusarium sp., respectively, each at varying cell densities (106–102 CFU/mL) and at distances of 20 mm. Expansion growth was measured daily until E. dermatitidis was completely covered by the competitor.
Results
Eight sample types were processed: two black yeast strains without treatment as control of viability and CFU count, and raw and sterilized compost, with or without spiking by Exophiala dermatitidis CBS 207.35 and Exophiala xenobiotica N1. Parameter of successful isolation was the number of CFUs after 7 or 14 days of incubation at room temperature for Methods 1 and 2 or after 2-month incubation at room temperature for Method 3. Results are summarized in Table 1.
Positive growth controls of E. dermatitidis CBS 207.35 when suspended from cell density 1 × 104 CFU/mL showed countless colonies for all three methods. Upon dilution 10× and 100 , CFUs were countable with all methods. Highly similar results were obtained with strain E. xenobiotica N1 with three methods.
Raw compost initial suspension and 10 × dilution yielded a large number of contaminant fungi rapidly covering the entire plates, without appearance of black fungi. At 1 × 102 dilution, the number of CFU was roughly estimated to be slightly higher with Method 1 than with Method 2. However, with Method 3 only 13 colonies of contaminants survived at the initial compost concentration, while with 10 and 100× dilution no fungal growth was observed.
Sterilized, un-spiked compost showed no growth. When spiked with CBS 207.35 or N1 at a concentration of 1 × 104, low numbers of Exophiala colonies were recovered with Methods 1 and 2, decreasing to zero at cell density 102 (Table 1). With Method 3, yields of the two tested black yeasts were in the same range as with pure suspensions.
Subsequently, samples of raw compost were spiked with strains CBS 207.35 or N1. At cell densities 1 ×104 and 1 ×103, countless colonies of fast-growing fungi appeared with Methods 1 and 2. At cell densities 1 ×102, numbers of fast-growing fungi were lower when testing CBS 207.35, and two colonies of E. dermatitidis appeared with Method 1. However, with Method 3, countless colonies of exclusively the target black yeasts CBS 207.35 and N1 were obtained at spiked concentration 1 x 103 and 1 x 104. At density 102, the targeted fungal CFUs were slightly lower, but still competitive with those obtained in Method 1 and Method 2. And only two colonies from the contaminated compost fungi were found in all experiments with Method 3.
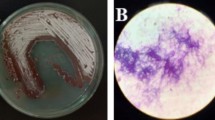
In the competition experiments, no zones of inhibition were observed between Aspergillus/Fusarium and Exophiala. Growth velocities of Aspergillus and Fusarium are 9 × and 7 × faster than that of Exophiala, respectively. When suspended at an equal cell density, Aspergillus and Fusarium overgrew Exophiala completely before the colonies of the latter had appeared. With Exophiala at a high cell density (106) and Aspergillus or Fusarium at low cell densities (103), black yeast colonies were visible and separate, but colonies of Aspergillus or Fusarium quickly dominated and covered Exophiala after 5 days (Fig. 2).
Results of competition experiment. aE. dermatitidis mixed with Aspergillus at the same cell density (106). bE. dermatitidis mixed with Fusarium at the same cell density (106). c High cell density of E. dermatitidis (106) mixed with low-cell-density Aspergillus (103). d High cell density of E. dermatitidis (106) mixed with low-cell-density Fusarium (103). e Inoculated at distances of 20 mm, E. dermatitidis and Aspergillus. f Inoculated at distances of 20 mm, E. dermatitidis and Aspergillus
Discussion
Exophiala is a genus of black yeasts known for its frequent occurrence in human infection [6]. The members of the genus are more likely to be opportunistic rather than pathogenic, as is judged from their environmental occurrence with life cycles where animal hosts do not play a significant role. As one of the possible explanations of their infective ability, their toxin management with an expansion of cytochrome P450 genes has been suggested [15]. This adds to their survival of conditions of osmotic stress and limited nutrient availability. Gostinčar et al. summarized these abilities under the term ‘polyextremotolerance’ [1]. Gueidan et al. and Teixeira et al. hypothesized that the evolutionary origin of this behavior might have coevolved with ants [16,17,18], which produce phenylacetic acid and other monoaromatic hydrocarbons from their exocrine glands as antibiotics in view of hygiene in their nests [19, 20]. Possibly, the genes required to deal with these toxic monoaromatic compounds have enabled management of a larger spectrum of related compounds, e.g., in creosote-treated railway sleepers [21], gasoline-polluted environments [22] or in air biofilters treating industrial exhaust gases containing volatile alkylbenzenes [23]. Decomposing cellulosic plant material is rarely inhabited by black yeasts, unless it is exceptionally rich in lipids, esters and alcohols [8] or tannins of degrading hardwood [24] and, hence, less easily accessible to common saprobes.
Extremotolerance can be understood as a way to escape competing microbes in the same habitat. Extreme habitats are usually poor in the number of species, but rich in the number of individuals surviving prevailing conditions. We analyzed compost as a highly competitive, non-extreme habitat by calculating survival rates via an extreme isolation step.
From raw compost, no black fungi were isolated with any of the three methods applied. When spiked with our model strains, Method 1 (conventional culturing) and Method 2 (oil flotation) yielded numerous cosmopolitan fungi present in the compost, while no black yeasts were recovered. Conventional culturing on general nutrient media allowed abundant growth of common compost fungi in Aspergillus and Fusarium. The used medium contained antibiotics to suppress bacterial growth, but was otherwise non-selective for fungi, in contrast to the classically applied media containing cycloheximide to specifically suppress fungal growth. This enabled to evaluate the recovery efficiency of our proposed Method 3 based on a solid-state-like enrichment in a toluene atmosphere.
Oil flotation isolation has been used by many researchers to isolate black yeast from environment [9, 13, 25, 26]. The principle of this method is hydrophobic interaction with hexadecane as an extraction agent, fungal cells collecting in the oil/water interface [13]. The latter authors obtained 107 black yeasts from only three samples of aromatic hydrocarbon-contaminated soil. The ratio is much higher than most preceding studies, presumably because the samples were already selective by their hydrocarbon content. In the present study, raw compost was used, a habitat containing numerous fast-growing competitors. Even after spiking with CBS 297.35, no black yeasts were recovered with conventional Methods 1 and 2. The competition experiment demonstrates that Exophiala is easily overgrown by rapidly expanding contaminants. These are inhibited in Method 3 where just toluene is present as source of carbon, enabling Exophiala to grow using its toluene degradation pathway [15]. Exophiala xenobiotica is known to degrade toluene (Prenafeta-Boldú et al. [11]). Some proteins that are associated with the toluene degradation pathway are also present in E. dermatitidis (https://genome.jgi.doe.gov/portal/).
Method 3 is based on the selective technique developed by Prenafeta-Boldú et al. [11] and explored further by Zhao et al. [10], but miniaturized in the present study for high-throughput purposes with the tested Methods 1 and 2 using un-spiked or spiked samples. Countless colonies of cosmopolitan fungi were observed and no or very few black yeast colonies were obtained, but with the Method 3 fast-growing fungi were efficiently suppressed and black yeasts grew prolifically. Therefore, it has effectively been proven that Method 3 can selectively isolate black fungi from nutrient-rich environmental samples, such as compost, which contain a wide diversity of fungi. When E. dermatitidis CBS 207.35 was mixed with sterilized compost, colony counts were lower than with pure suspensions, as well as in relation to those from the fungus E. xenobiotica N1. Possibly, compost contains compounds or secondary metabolites produced by other microbes that inhibit growth of E. dermatitidis.
In conclusion, the main reason why black yeasts are difficult to isolate from the environment seems to be their slow growth rate and their inability to inhibit fungal competitors under standard laboratory conditions. They are easily suppressed and overgrown by common fast-growing fungi in under non-limiting conditions for microbial growth. Therefore, conventional culturing has a low efficiency for this particular group of fungi, while creating selective pressure based on the direct exposure to vapors of toxic alkylbenzenes proves to be highly effective. High-throughput isolation of black yeasts may help to understand the presence and role of these fungi in natural and polluted environments and to uncover potential routes of infection and their significance to public health.
References
Gostinčar C, Zajc J, Lenassi M, Plemenitaš A, de Hoog GS, Al-Hatmi AM, et al. Fungi between extremotolerance and opportunistic pathogenicity on humans. Fungal Divers. 2018;93(1):195–213. https://doi.org/10.1007/s13225-018-0414-8.
Chowdhary A, Perfect J, de Hoog GS. Black molds and melanized yeasts pathogenic to humans. Cold Spring Harbor Perspect Med. 2015;5(8):a019570.
Prenafeta-Boldú FX, Roca N, Villatoro C, Vera L, de Hoog GS. Prospective application of melanized fungi for the biofiltration of indoor air in closed bioregenerative systems. J Hazard Mater. 2019;361:1–9. https://doi.org/10.1016/j.jhazmat.2018.08.059.
Gostinčar C, Muggia L, Grube M. Polyextremotolerant black fungi: oligotrophism, adaptive potential, and a link to lichen symbioses. Front Microbiol. 2012;3:390. https://doi.org/10.3389/fmicb.2012.00390.
Prenafeta-Boldu FX, Summerbell R, de Hoog GS. Fungi growing on aromatic hydrocarbons: biotechnology’s unexpected encounter with biohazard? FEMS Microbiol Rev. 2006;30(1):109–30. https://doi.org/10.1111/j.1574-6976.2005.00007.x.
De Hoog GS, Vicente VA, Najafzadeh M, Harrak M, Badali H, Seyedmousavi S. Waterborne Exophiala species causing disease in cold-blooded animals. Persoonia Mol Phylogeny Evol Fungi. 2011;27:46–72. https://doi.org/10.3767/003158511X614258.
Hyde KD, Al-Hatmi AM, Andersen B, Boekhout T, Buzina W, Dawson TL, et al. The world’s ten most feared fungi. Fungal Divers. 2018;93(1):161–94.
Nascimento MM, Vicente VA, Bittencourt JV, Gelinski JML, Prenafeta-Boldú FX, Romero-Güiza M, et al. Diversity of opportunistic black fungi on babassu coconut shells, a rich source of esters and hydrocarbons. Fungal Biol. 2017;121(5):488–500. https://doi.org/10.1016/j.funbio.2017.01.006.
Vicente VA, Attili Angelis D, Queiróz-Telles F, Pizzirani-Kleiner AA. Isolation of herpotrichiellacious fungi from the environment. Braz J Microbiol. 2001;32(1):47–51.
Zhao J, Zeng J, de Hoog GS, Attili-Angelis D, Prenafeta-Boldú FX. Isolation and identification of black yeasts by enrichment on atmospheres of monoaromatic hydrocarbons. Microb Ecol. 2010;60(1):149–56.
Prenafeta-Boldú FX, Andrea K, Luykx DM, Heidrun A, van Groenestijn JW. Isolation and characterisation of fungi growing on volatile aromatic hydrocarbons as their sole carbon and energy source. Mycol Res. 2001;105(4):477–84. https://doi.org/10.1017/S0953756201003719.
Badali H, Prenafeta-Boldu FX, Guarro J, Klaassen CH, Meis JF, de Hoog GS. Cladophialophora psammophila, a novel species of Chaetothyriales with a potential use in the bioremediation of volatile aromatic hydrocarbons. Fungal Biol. 2011;115(10):1019–29. https://doi.org/10.1016/j.funbio.2011.04.005.
Satow M, Attili-Angelis D, de Hoog GS, Attili-Angelis D, Vicente VA. Selective factors involved in oil flotation isolation of black yeasts from the environment. Stud Mycol. 2008;61:157–63. https://doi.org/10.3114/sim.2008.61.16.
Hartmans S, Tramper J. Dichloromethane removal from waste gases with a trickle-bed bioreactor. Bioprocess Eng. 1991;6(3):83–92.
Moreno LF, Ahmed AA, Brankovics B, Cuomo CA, Menken SBJ, Taj-Aldeen SJ, et al. Genomic understanding of an infectious brain disease from the desert. G3: Genes Genomes Genet. 2018;3:909–22. https://doi.org/10.1534/g3.117.300421.
Gueidan C, Ruibal C, de Hoog GS, Schneider H. Rock-inhabiting fungi originated during periods of dry climate in the late Devonian and middle Triassic. Fungal Biol. 2011;115(10):987–96. https://doi.org/10.1016/j.funbio.2011.04.002.
Teixeira MM, Moreno LF, Stielow B, Muszewska A, Hainaut M, Gonzaga L, et al. Exploring the genomic diversity of black yeasts and relatives (Chaetothyriales, Ascomycota). Stud Mycol. 2017;86:1–28. https://doi.org/10.1016/j.simyco.2017.01.001.
Nepel M, Voglmayr H, Schönenberger J, Mayer VE. High diversity and low specificity of Chaetothyrialean fungi in carton galleries in a Neotropical ant–plant association. PLoS ONE. 2014;9(11):e112756. https://doi.org/10.1371/journal.pone.0112756.
Fernández-Marín H, Zimmerman JK, Rehner SA, Wcislo WT. Active use of the metapleural glands by ants in controlling fungal infection. Proc R Soc Lond B: Biol Sci. 2006;273(1594):1689–95. https://doi.org/10.1098/rspb.2006.3492.
Fernández-Marín H, Nash DR, Higginbotham S, Estrada C, van Zweden JS, d’Ettorre P, et al. Functional role of phenylacetic acid from metapleural gland secretions in controlling fungal pathogens in evolutionarily derived leaf-cutting ants. Proc R Soc B. 1807;2015(282):20150212. https://doi.org/10.1098/rspb.2015.0212.
Döğen A, Ilkit M, de Hoog GS. Black yeast habitat choices and species spectrum on high altitude creosote-treated railway ties. Fungal Biol. 2013;117(10):692–6.
Isola D, Selbmann L, de Hoog GS, Fenice M, Onofri S, Prenafeta-Boldú FX, et al. Isolation and screening of black fungi as degraders of volatile aromatic hydrocarbons. Mycopathologia. 2013;175(5–6):369–79. https://doi.org/10.1016/j.funbio.2013.07.006.
Woertz J, Kinney K, McIntosh N, Szaniszlo P. Removal of toluene in a vapor-phase bioreactor containing a strain of the dimorphic black yeast Exophiala lecanii-corni. Biotechnol Bioeng. 2001;75(5):550–8.
Vicente V, Attili-Angelis D, Pie M, Queiroz-Telles F, Cruz L, Najafzadeh M, et al. Environmental isolation of black yeast-like fungi involved in human infection. Stud Mycol. 2008;61:137–44. https://doi.org/10.3114/sim.2008.61.14.
Marques SG, Silva CDMP, Saldanha PC, Rezende MA, Vicente VA, Queiroz-Telles F, et al. Isolation of Fonsecaea pedrosoi from the shell of the babassu coconut (Orbignya phalerata Martius) in the Amazon region of Maranhão Brazil. Nippon Ishinkin Gakkai Zasshi. 2006;47(4):305–11.
Iwatsu T, Miyaji M, Okamoto S. Isolation of Phialophora verrucosa and Fonsecaea pedrosoi from nature in Japan. Mycopathologia. 1981;75(3):149–58.
Acknowledgements
Francesc Prenafeta-Boldú is member of the Consolidated Research Group TERRA (Ref. 2017 SGR 1290). The support of the CERCA Programme (Generalitat de Catalunya) is also acknowledged. We would like to thank China Scholarship Council for funding support. We also would like to thank Hein van der Lee and Marlou Tehupeiory-Kooreman for the experimental support.
Funding
This work was supported by Guizhou Scientific Plan Project [(2019) 2873]; Excellent Youth Talent Training Project of Guizhou Province [(2017) 5639]; Guiyang Science and Technology Project [(2017) No.5-19]; Talent Base Project of Guizhou Province, China [FCJD2018-22]; Research Fund of Education Bureau of Guizhou Province, China [(2018) 481].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Macit Ilkit.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Quan, Y., van den Ende, B.G., Shi, D. et al. A Comparison of Isolation Methods for Black Fungi Degrading Aromatic Toxins. Mycopathologia 184, 653–660 (2019). https://doi.org/10.1007/s11046-019-00382-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-019-00382-3