Abstract
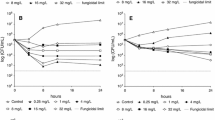
Currently echinocandins are recommended in Candida peritonitis and pleuritis. We determined micafungin killing rates (k values) at therapeutic concentrations (0.25–2 mg/L) in RPMI-1640 with and without 10 and 30% serum mimicking in vivo conditions against six Candida species isolated from peritoneal and pleural fluid. In RPMI-1640, micafungin was fungicidal against C. glabrata, C. krusei and C. kefyr within 2.27 ± 10.68, 2.69 ± 10.29 and 3.10 ± 4.41 h, respectively, while was fungistatic against C. albicans, C. tropicalis and C. parapsilosis. In 10% serum, ≥ 0.25, ≥ 0.5, ≥ 0.5 and ≥ 1 mg/L micafungin produced positive k values (killing) for all C. albicans, C. glabrata, C. kefyr and C. krusei, respectively. In 30% serum, 2 mg/L micafungin produced killing against all C. albicans, C. glabrata and C. kefyr isolates, but was ineffective against C. krusei, C. parapsilosis and 2 of 3 C. tropicalis. Micafungin exposure should be increased against non-albicans species to eradicate fungi from peritoneal and pleural cavities.

Similar content being viewed by others
References
Perlin DS. Echinocandin resistance, susceptibility testing and prophylaxis: implications for patient management. Drugs. 2014;74:1573–85.
Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1–50.
Nasar A, Ryan L, Frei CR, Cota JM, Wiederhold NP. Influence of serum and albumin on echinocandin in vitro potency and pharmacodynamics. Curr Fungal Infect Rep. 2013;7:89–95.
Saleh Q, Kovács R, Kardos G, Gesztelyi R, Kardos T, Bozó A, et al. Decreased killing activity of micafungin against Candida guilliermondii, Candida lusitaniae, and Candida kefyr in the presence of human serum. Microb Drug Resist. 2017;23:764–70.
Földi R, Szilágyi J, Kardos G, Berényi R, Kovács R, Majoros L. Effect of 50% human serum on the killing activity of micafungin against eight Candida species using time-kill methodology. Diagn Microbiol Infect Dis. 2012;73:338–42.
Kovács R, Gesztelyi R, Berényi R, Domán M, Kardos G, Juhász B, et al. Killing rates exerted by caspofungin in 50% serum and its correlation with in vivo efficacy in a neutropenic murine model against Candida krusei and C. inconspicua. J Med Microbiol. 2014;63:186–94.
Földi R, Kovács R, Gesztelyi R, Kardos G, Berényi R, Juhász B, et al. Comparison of in vitro and in vivo efficacy of caspofungin against Candida parapsilosis, C. orthopsilosis, C. metapsilosis and C. albicans. Mycopathologia. 2012;174:311–8.
Szilágyi J, Földi R, Bayegan S, Kardos G, Majoros L. Effect of nikkomycin Z and 50% human serum on the killing activity of high-concentration caspofungin against Candida species using time-kill methodology. J Chemother. 2012;24:18–25.
Kovács R, Saleh Q, Bozó A, Tóth Z, Gesztelyi R, Kardos T, Kardos G, Takacs I, Majoros L. Killing activity of micafungin against Candida albicans, C. dubliniensis and Candida africana in the presence of human serum. Mycopathologia. 2017;182:979–87.
Yamada N, Kumada K, Kishino S, Mochizuki N, Ohno K, Ogura S. Distribution of micafungin in the tissue fluids of patients with invasive fungal infections. J Infect Chemother. 2011;17:731–4.
Grau S, Luque S, Campillo N, Samsó E, Rodríguez U, García-Bernedo CA, et al. Plasma and peritoneal fluid population pharmacokinetics of micafungin in post-surgical patients with severe peritonitis. J Antimicrob Chemother. 2015;70:2854–61.
García-de-Lorenzo A, Luque S, Grau S, Agrifoglio A, Cachafeiro L, Herrero E, et al. Comparative population plasma and tissue pharmacokinetics of micafungin in critically ill patients with severe burn injuries and patients with complicated intra-abdominal infection. Antimicrob Agents Chemother. 2016;60:5914–21.
Zhao Y, Prideaux B, Nagasaki Y, Lee MH, Chen PY, Blanc L, et al. Unraveling drug penetration of echinocandin antifungals at the site of infection in an intra-abdominal abscess model. Antimicrob Agents Chemother. 2017;61:e01009–17.
Moriyama B, Ditullio M, Wilson E, Henning SA, Penzak SR, Danner RL, et al. Pharmacokinetics of anidulafungin in pleural fluid during the treatment of a patient with Candida empyema. Antimicrob Agents Chemother. 2011;55:2478–80.
Clinical and Laboratory Standards Institute. 2008: Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, 3rd ed. M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA.
Huang LL, Xia HH, Zhu SL. Ascitic fluid analysis in the differential diagnosis of ascites: focus on cirrhotic ascites. J Clin Transl Hepatol. 2014;2:58–64.
Ferreiro L, Porcel JM, Valdés L. Diagnosis and management of pleural transudates. Arch Bronconeumol. 2017;53:629–36.
Porcel JM, Light RW. Diagnostic approach to pleural effusion in adults. Am Fam Physician. 2006;73:1211–20.
Tarn AC, Lapworth R. Biochemical analysis of ascitic (peritoneal) fluid: what should we measure? Ann Clin Biochem. 2010;47:397–407.
Wahidi MM, Willner DA, Snyder LD, Hardison JL, Chia JY, Palmer SM. Diagnosis and outcome of early pleural space infection following lung transplantation. Chest. 2009;135:484–91.
Davies HE, Davies RJ, Davies CW, BTS Pleural Disease Guideline Group. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii41–53.
Srinivasnakshatri VK, Subramani P, Venkateshwaraprasad KN, Varma P. A fatal case of fungal empyema due to Candida krusei and Candida tropicalis: a rare occurrence with an atypical presentation. J Clin Diagn Res. 2014;8:DD01–2.
Pfaller MA, Castanheira M, Diekema DJ, Messer SA, Jones RN. Triazole and echinocandin MIC distributions with epidemiological cutoff values for differentiation of wild-type strains from non-wild-type strains of six uncommon species of Candida. J Clin Microbiol. 2011;49:3800–4.
Vergidis P, Clancy CJ, Shields RK, Park SY, Wildfeuer BN, Simmons RL, et al. Intra-abdominal candidiasis: the importance of early source control and antifungal treatment. PLoS ONE. 2016;11:e0153247.
Bassetti M, Righi E, Ansaldi F, Merelli M, Scarparo C, Antonelli M, Garnacho-Montero J, et al. A multicenter multinational study of abdominal candidiasis: epidemiology, outcomes and predictors of mortality. Intensive Care Med. 2015;41:1601–10.
Montravers P, Mira JP, Gangneux JP, Leroy O, Lortholary O, The AmarCand study group. A multicentre study of antifungal strategies and outcome of Candida spp. peritonitis in intensive-care units. Clin Microbiol Infect. 2011;17:1061–7.
Ko SC, Chen KY, Hsueh PR, Luh KT, Yang PC. Fungal empyema thoracis: an emerging clinical entity. Chest. 2000;117:1672–8.
Lin HS, Chao CM, Lin WT, Lai CC. Candida empyema thoracis at a hospital in Taiwan. Surg Infect (Larchmt). 2014;15:540–3.
Nigo M, Vial MR, Munita JM, Jiang Y, Tarrand J, Jimenez CA, et al. Fungal empyema thoracis in cancer patients. J Infect. 2016;72:615–21.
Pemán J, Aguilar G, Valía JC, Salavert M, Navarro D, Zaragoza R, et al. Jávea consensus guidelines for the treatment of Candida peritonitis and other intra-abdominal fungal infections in non-neutropenic critically ill adult patients. Rev Iberoam Micol. 2017;34:130–42.
Bordallo-Cardona MÁ, Escribano P, Marcos-Zambrano LJ, Díaz-García J, de la Pedrosa EG, Cantón R, et al. Low and constant micafungin concentrations may be sufficient to lead to resistance mutations in FKS2 gene of Candida glabrata. Med Mycol. 2017. https://doi.org/10.1093/mmy/myx124.
Shields RK, Nguyen MH, Press EG, Clancy CJ. Abdominal candidiasis is a hidden reservoir of echinocandin resistance. Antimicrob Agents Chemother. 2014;58:7601–5.
Fekkar A, Meyer I, Brossas JY, Dannaoui E, Palous M, Uzunov M, et al. Rapid emergence of echinocandin resistance during Candida kefyr fungemia treatment with caspofungin. Antimicrob Agents Chemother. 2013;57:2380–2.
Xiao M, Fan X, Hou X, Chen SC, Wang H, Kong F, et al. Clinical characteristics of the first cases of invasive candidiasis in China due to pan-echinocandin-resistant Candida tropicalis and Candida glabrata isolates with delineation of their resistance mechanisms. Infect Drug Resist. 2018;11:155–61.
Acknowledgements
L. Majoros received conference travel grants from MSD, Astellas and Pfizer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Ana Alastruey-Izquierdo.
Rights and permissions
About this article
Cite this article
Tóth, Z., Kardos, T., Kovács, R. et al. Comparison of Killing Activity of Micafungin Against Six Candida Species Isolated from Peritoneal and Pleural Cavities in RPMI-1640, 10 and 30% Serum. Mycopathologia 183, 905–912 (2018). https://doi.org/10.1007/s11046-018-0302-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-018-0302-5