Abstract
Invasive mucormycosis in immunocompromised children is a life-threatening fungal infection. We report a case of a 7-year-old girl treated for acute lymphoblastic leukaemia complicated by disseminated mucormycosis during induction therapy. Microscopic examination of surgically removed lung tissue revealed wide, pauci-septate hyphae suggesting a Mucorales infection. This diagnosis was confirmed immunohistochemically and by PCR analysis followed by a final identification of Cunninghamella sp. The patient was treated successfully with surgical debridement and antifungal combination therapy with amphotericin B, caspofungin and isavuconazole. The use of isavuconazole in a child was not previously reported. Additionally, case reports concerning pulmonary mucormycoses in paediatric population published after 2010 were reviewed. Nineteen out of 26 identified patients suffered from haematological diseases. Reported mortality reached 38.5%. By the fact of rising morbidity, unsatisfactory results of treatment and remaining high mortality of mucormycoses in immunocompromised patients, new therapeutic options are warrant. Isavuconazole, with its broad-spectrum activity, good safety profile and favourable pharmacokinetics, is a promising drug. However, further studies are necessary to confirm positive impact of isavuconazole on mucormycosis treatment in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mucormycosis was first described by Furbringer in 1876 [1]. Almost 100 years later Baker was still characterizing it as most acutely fatal mycosis [2]. In recent years, raising numbers of fungal infections caused by Mucorales have been reported [3]. Despite awareness of the problem, new antifungal drugs and more precise diagnostics, morbidity and mortality due to mucormycosis are still unacceptable [4].
Isavuconazole is a broad-spectrum triazole agent targeting the CYP51A enzyme important for ergosterol synthesis and cell membrane formation. It has in vitro and in vivo activity against Mucorales [5], but unfortunately, this is not yet registered for children.
In this report, we describe the successful treatment of mucormycosis in a child with acute lymphoblastic leukaemia (ALL), which is the first article describing usage of isavuconazole in a child.
Case Report
A 7-year-old girl treated for intermediate risk ALL (Protocol ALL IC BFM 2009) obtained complete remission after 33 days of induction therapy. The induction chemotherapy was complicated by myelosuppression, tubulopathy, cholestasis and insulin-dependent diabetes. Primary antifungal prophylaxis was not implemented.
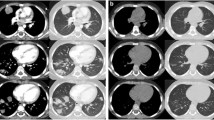
On the 38 day of ALL therapy, the patient experienced febrile neutropenia and C-reactive protein (CRP) was elevated (65 mg/L). Chemotherapy was interrupted and empiric antibiotic therapy (cefepime, vancomycin and clarithromycin) was implemented, but with no improvement of the clinical condition. Chest computed tomography (CT) scan showed local infiltration in the left lung (52 × 44 mm), two focal lesions in the right lung and left-sided pleural effusion (Fig. 1a). Due to suspicion of invasive aspergillosis, despite negative galactomannan, pre-emptive antifungal therapy with voriconazole was started and granulocyte colony-stimulating factor (GCSF) was added as supportive care. After 9 days, CRP was further elevated (140 mg/L). Follow-up CT scan showed progression of the pulmonary infection with infiltration of the II intercostal space (Fig. 1b). Because of the rapid progression with soft tissues involvement, in spite of implemented treatment, mucormycosis was suspected as differential diagnosis. Voriconazole was discontinued, and combination antifungal treatment with amphotericin B lipid complex (ABLC) and caspofungin was introduced.
Chest computed tomography: a local infiltration in the left lung, two focal lesions in the right lung, and left-sided pleural effusion; b follow-up image after 9 days showing CT progression with infiltration of the II intercostal space; c control image after 8 months of antifungal treatment showing complete regression
The lesions were surgically removed. Microscopic examination revealed areas of chronic, necrotizing, pyogranulomatous pneumonia containing wide and sparsely septate hyphae which had also invaded vessels (Fig. 2a). The hyphae morphology was suggestive of a Mucorales infection. The culture of the infected tissue and GM from the pleural fluid were both negative. Formalin-fixed paraffin-embedded tissue was referred to Statens Serum Institut (SSI) in Copenhagen and to the Faculty of Health and Medical Sciences at the University of Copenhagen for further diagnostics.
Microscopic images: a pulmonary mucormycosis with angioinvasion. The hyphae are infrequent septate, show irregular contours, and a random and haphazard pattern of branching. Grocott, Obj. × 40; b pulmonary mucormycosis. Immunostaining of hyphae revealed a strong staining only with monoclonal antibodies (Mab WSSA-RA-1) reacting specifically with genera of the order Mucorales. Obj. × 40
Immunohistochemical staining was used to identify the aetiological agent. Sections were mounted on adhesive slides (Superfrost R Plus; Menzel-Glaser, Germany) and kept at 4 °C until processed. Primary reagents for immunostaining included specific monoclonal and polyclonal antibodies reacting with genera of the order Mucorales, and species of the genera Aspergillus, Candida, Geotrichum, Fusarium and Scedosporium, respectively [6, 7]. The detection system PowerVision + (Part No. DPVB + 110AEC; Immuno Vision Technologies Co. USA) was used for signal amplification. Sections were counterstained in Meyer’s haematoxylin for 10 s. and washed for 1 min. in tap water and 4 min. in distilled water. Finally, sections were mounted with glycerol gelatin. To ensure specific reactivity of antibodies, the sections were run in parallel with sections from laboratory animals experimentally infected with homologous and heterologous fungi. Moreover, in all series of stainings, also the following negative controls were run: (1) without a primary reagents and (2) with substitution of the primary antiserum or monoclonal antibody of identical isotypes, respectively, raised towards nonsense antigens. Immunostaining of hyphae revealed a strong staining only with monoclonal antibodies (Mab WSSA-RA-1) reacting specifically with genera of the order Mucorales [6] (Fig. 2b).
Molecular analyses were accomplished by PCR and Sanger sequencing as well as a microbiome analysis. Two lung tissue samples were deparaffinized using conventional Neo-Clear, a xylene substitute (Merck) and methanol. Tissue was lysed overnight with proteinase K and DNA extracted using the Qiacube (Qiagen) with elution in 100 µL. PCR was performed using pan-fungal primers V9G and LS266 [8] spanning the internal transcribed spacer regions (ITS1 and ITS2), and DNA sequences were analysed using online tools; NCBI BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and pairwise sequence alignment available through the Westerdijk Fungal Biodiversity Institute (http://www.westerdijkinstitute.nl/Collections/BioloMICSSequences.aspx?file=all).
Microbiome analysis was based on a next-generation sequencing platform using 3 primer sets targeting the hypervariable regions V3–V4 of the 18SrDNA gene, as previously described [9]. Sanger sequencing did not provide high-quality sequences but best similarity (> 90%) and unambiguously to Cunninghamella species. Microbiome analyses generated 12,000 (> 20%) and 42,000 reads (> 60%) clustered as Cunninghamella species, of which some sequences were independently validated by alignment and full length sequence BLAST search.
Despite continuation of combination antifungal therapy, the follow-up chest CT scan showed progression in the right lung 14 days after surgery. Ultrasound and MRI examination of the abdomen showed focal changes in the liver, suggesting fungal infiltration. Additionally, massive generalized secondary haemochromatosis was described in magnetic resonance imaging (MRI) of the liver. Ferritin serum level reached 8000 ng/ml (upper limit 360 ng/ml).
Posaconazole was added as the salvage therapy, but stopped after 3 days due to the patient’s problems with taking oral medication. The intravenous posaconazole formulation is not available in Poland. Because of the resistant mucormycosis, combination therapy with ABLC, caspofungin and isavuconazole was implemented.
Ethical approval from independent ethics committee was obtained and parents provided written informed consent for off-label isavuconazole administration. Dosing of isavuconazole as for adults with therapeutic drug monitoring (TDM) was prescribed. Blood samples were collected and sent to Switzerland to Basilea Pharmaceutica International Ltd. for testing. For the first 48 h, the patient received loading dose: 6 administrations of 200 mg of isavuconazole intravenously every 8 h, followed by isavuconazole 200 mg once daily. TDM was done on days: 3, 5, 7 and 28 of the isavuconazole therapy reaching trough levels of 2.92, 2.16, 2.1 and 2.45 ug/ml, respectively. It was decided to increase the dose of isavuconazole to 2 × 200 mg to further increase exposure given the fact that Mucorales are somewhat less susceptible than Aspergillus. TDM was done again after 1, 2, 3 and 4 weeks reaching isavuconazole trough levels of 6.07, 3.07, 5.04 and 4.28 ug/ml, respectively. The patient received isavuconazole treatment for 147 days intravenously and subsequently for 99 days orally. Isavuconazole was used in combination treatment for 101 days and then as monotherapy; no side effects were observed. Chemotherapeutic protocol was continued during the period of antifungal treatment.
The follow-up chest CT and abdominal ultrasound examination confirmed complete regression (Fig. 1c). When intensive chemotherapy was terminated, the isavuconazole treatment was discontinued, and secondary prophylaxis with posaconazole was started. During a 10-months observation period from completing isavuconazole treatment, the patient has been well, in the first complete haematological remission, during maintenance chemotherapy.
Discussion
Mucormycosis and especially infections caused by Cunninghamella are rare but carry one of the highest mortality rates among all mycoses. The most significant risk factors are: prolonged neutropenia, former broad-spectrum antibiotic therapy and diabetes including steroid-induced [4]. Review of the paediatric pulmonary mucormycosis reports published since 2010 based on PubMed and Cochrane databases showed that mucormycosis risk factors were: neutropenia (61.5% patients), broad-spectrum antibiotic therapy (35%), previous treatment with voriconazole (31%) and diabetes (27%) (Table 1) [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. A total of 26 patients were identified: 19 children suffered from haematological disease, 5 had diabetes, one had osteosarcoma and one was diagnosed after a near-drowning incident. In 6 patients, mucormycosis occurred after allogeneic haematopoietic stem cell transplantation (allo-HSCT). Most children had more than one risk factor. The overall mortality was 38.5% (11 patients died, status of one patient is unknown). Despite progress in diagnosis and management, rates of successful outcome are still low.
Unappreciated but significant issue in haematological patients is iron overload resulting from multiple blood transfusions. The link between iron availability and the risk of mucormycosis is well known [26]. There are two children with iron overload in the review (Table 1): one was diagnosed with haemochromatosis before allo-HSCT and received deferoxamine, and the other patient was iron overloaded at the moment of diagnosis of mucormycosis and received deferasirox as adjuvant therapy [18, 19]. It is worth mentioning that there were high hopes for use of iron chelators together with antifungal therapy in patients with mucormycosis. Studies with the animal models showed that deferasirox administered to diabetic ketoacidotic or neutropenic mice with mucormycosis, significantly improved survival and decreased tissue fungal burden with a similar efficacy to liposomal amphotericin B (LAMB) [27]. Unfortunately, a double-blinded, randomized, placebo-controlled, phase II study (the DEFEAT Mucor study) showed higher mortality rate in patients treated with adjunctive deferasirox in comparison with those treated with placebo [28]. Therefore, ECIL-6 guidelines recommend against use of deferasirox in the therapy of mucormycosis [29].
Present guidelines in mucormycosis therapy recommend antifungal treatment, surgical debridement and improvement of underlying conditions such as usage of hematopoietic growth factor in case of neutropenia. According to ECIL-6 guidelines, high doses of LAMB (5 mg/kg/day) or ABLC are recommended as a first line therapy of mucormycosis. Posaconazole is recommended as alternative and salvage therapy. Combination antifungal therapy with ABLC and caspofungin or posaconazole can also be considered for salvage therapy of mucormycosis [29]. In the literature review (Table 1), different antifungal drugs and their combinations were implemented. Monotherapy was used in 13 cases; other patients received combination therapy (eight children with two drugs and four with three drugs). Additionally, in 17 cases surgical debridement was performed (70.5% of them survived), as in our patient. In contrast, only one-third of patients without surgical intervention survived. Two patients underwent stem cell transplantation as adjuvant therapy to resolve prolonged neutropenia.
Our patient received combination therapy with ABLC, caspofungin and isavuconazole. To best of our knowledge, this is the first description of both isavuconazole therapy in children and isavuconazole in combination treatment. Salvage therapy with isavuconazole was implemented due to the rapid progression of disseminated infection. Despite the surgery and usage of prior combination therapy with ABLC and caspofungin together with neutrophil growth factor, the infection disseminated to the liver. Although there is no recommendation for isavuconazole in mucormycosis treatment in ECIL-6, the VITAL study showed isavuconazole activity against mucormycosis with efficacy similar to amphotericin B with good tolerance [30].
Isavuconazole is a new broad-spectrum antifungal drug, which was approved in 2016 in USA and in Europe for treatment of invasive aspergillosis and mucormycosis when amphotericin B is inappropriate. Isavuconazole is a second-generation triazole, administered as the prodrug. It displays excellent bioavailability after oral administration (98%). In contrast to voriconazole and posaconazole, isavuconazole is food independent enabling the intravenous and oral formulations to be used interchangeably [31, 32]. Our patient was not eligible for oral medication due to severe psychogenic nausea and vomiting; for this reason, she received such a long intravenous treatment. The SECURE study showed reduction in side effects compared to voriconazole (17%) leading to shorter hospitalization, shorter interruptions of chemotherapy and improved life quality of the patients [33].
Need for TDM of isavuconazole is controversial. Some authors suggest that it could be indicated in selected clinical cases [34]. Up to now, there was no dose-finding study in mucormycosis, and adult patients were administered the same dose of isavuconazole as was used for the treatment of invasive aspergillosis. Moreover, there are no algorithms on how to adjust the dose of isavuconazole depending on the plasma concentration. (In the VITAL study in patients with invasive mould infections, it was in the range of 3–4 ug/ml [30].) The patient described herein achieved the desired plasma concentration after increasing the dose from 200 mg to 2 × 200 mg per day, and drug plasma concentration was maintained in the higher range because of the disseminated Cunninghamella infection together with the successful outcome, suggesting that TDM was helpful in this case.
Conclusions
Isavuconazole may be an effective treatment option for disseminated mucormycosis in patients who have been found unresponsive to other therapies, especially in combination treatment. Despite the controversies with necessity of TDM, in the reported patient it was a reason to intensify the antifungal treatment. However, current knowledge about isavuconazole use in children is still limited and future studies are necessary; one has been conducted in USA since October 2017 (ClinicalTrials.gov Identifier: NCT03241550).
References
Fürbringer P. Beobachtungen über Lungenmycose beim Menschen. Arch für Pathol Anat und Physiol und für Klin Med. 1876;66:330–65.
Baker RD. Resectable mycotic lesions and acutely fatal mycoses. J Am Med Assoc. 1952;150:1579–81.
Guinea J, Escribano P, Vena A, et al. Increasing incidence of mucormycosis in a large Spanish hospital from 2007 to 2015: epidemiology and microbiological characterization of the isolates. PLoS ONE. 2017;12:1–10.
Pana ZD, Seidel D, Skiada A, et al. Invasive mucormycosis in children: an epidemiologic study in European and non-European countries based on two registries. BMC Infect Dis. 2016;16:667.
Arendrup MC, Jensen RH, Meletiadis J. In vitro activity of isavuconazole and comparators against clinical isolates of the Mucorales order. Antimicrob Agents Chemother. 2015;12:7735–42.
Jensen HE, Salonen J, Ekfors TO. The use of immunohistochemistry to improve sensitivity and specificity in the diagnosis of systemic mycoses in patients with haematological malignancies. J Pathol. 1997;181:100–5.
Jensen HE, Schønheyder HC, Hotchi M, Kaufman L. Diagnosis of systemic mycoses by specific immunohistochemical tests. APMIS. 1996;104:241–58.
Irinyi L, Lackner M, de Hoog GS, Meyer W. DNA barcoding of fungi causing infections in humans and animals. Fungal Biol. 2016;120:125–36.
Ring HC, Thorsen J, Saunte DM, et al. The follicular skin microbiome in patients with hidradenitis suppurativa and healthy controls. JAMA Dermatol. 2017;153:897–905.
Roux BG, Méchinaud F, Gay-Andrieu F, et al. Successful triple combination therapy of disseminated Absidia corymbifera infection in an adolescent with osteosarcoma. J Pediatr Hematol. 2010;32:131–3.
Garbino J, Myers C, Ambrosioni J, Gumy-Pause F. Report of a successful treatment of pulmonary Cunninghamella bertholletiae infection with liposomal amphotericin and posaconazole in a child with GvHD and review of the literature. J Pediatr Hematol Oncol. 2010;32:85–7.
Weng TF, Ho MW, Lin HC, et al. Successful treatment of disseminated mixed invasive fungal infection after hematopoietic stem cell transplantation for severe aplastic anemia. Pediatr Transplant. 2012;16:E35–8.
Däbritz J, Attarbaschi A, Tintelnot K, et al. Mucormycosis in paediatric patients: demographics, risk factors and outcome of 12 contemporary cases. Mycoses. 2011;54:785–8.
Phulpin-Weibel A, Rivier A, Leblanc T, et al. Focus on invasive mucormycosis in paediatric haematology oncology patients: a series of 11 cases. Mycoses. 2013;56:236–40.
José González-Abad MJ, Alonso Sanz M. Zygomycosis in children: disseminated infection caused by Cunninghamella bertholletiae. Arch Bronconeumol. 2013;49:35.
Guymer C, Khurana S, Suppiah R, et al. Successful treatment of disseminated mucormycosis in a neutropenic patient with T-cell acute lymphoblastic leukaemia. BMJ Case Rep. 2013;31:bcr2013009577.
Gupta A, Jain S, Agrawal C, Kapoor G. Successful outcome of mucormycosis in two children on induction therapy for acute lymphoblastic leukemia. Indian J Med Paediatr Oncol. 2013;34:313–6.
Ye B, Yu D, Zhang X, et al. Disseminated Rhizopus microsporus infection following allogeneic hematopoietic stem cell transplantation in a child with severe aplastic anemia. Transpl Infect Dis. 2013;15:E216–23.
Carceller F, Onoro G, Buitrago MJ, et al. Cunninghamella bertholletiae infection in children: review and report of 2 cases with disseminated infection. J Pediatr Hematol Oncol. 2014;36:e109–14.
Dayal D, Jain P, Kumar R, et al. Clinical spectrum and outcome of invasive filamentous fungal infections in children with type 1 diabetes: north Indian experience. Clin Pediatr Endocrinol. 2015;24:51–7.
Gerlach MM, Lippmann N, Kobelt L, et al. Possible pulmonary Rhizopus oryzae infection in a previously healthy child after a near-drowning incident. Infection. 2016;44:361–4.
Suzuki D, Kobayashi R, Hori D, et al. Stem cell transplantation for acute myeloid leukemia with pulmonary and cerebral mucormycosis. Pediatr Int. 2016;58:569–72.
Seo YM, Hwang-Bo S, Kim SK, et al. Fatal systemic adenoviral infection superimposed on pulmonary mucormycosis in a child with acute leukemia: a case report. Med (Baltim). 2016;95:e5054.
Malkan AD, Wahid FN, Rao BN, Sandoval JA. Aggressive Cunninghamella pneumonia in an adolescent. J Pediatr Hematol Oncol. 2014;36:581–2.
Wang XM, Guo LC, Xue SL, Chen YB. Pulmonary mucormycosis: a case report and review of the literature. Oncol Lett. 2016;11:3049–53.
Ibrahim AS. Host-iron assimilation: pathogenesis and novel therapies of mucormycosis. Mycoses. 2014;57:13–7.
Ibrahim AS, Gebermariam T, Fu Y, et al. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J Clin Investig. 2007;117:2649–57.
Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The deferasirox-AmBisome therapy for mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67:715–22.
Tissot F, Agrawal S, Pagano L, et al. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica. 2017;102:433–44.
Marty FM, Ostrosky-Zeichner L, Cornely OA, et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16:828–37.
Donnelley MA, Zhu ES, Thompson GR. Isavuconazole in the treatment of invasive aspergillosis and mucormycosis infections. Infect Drug Resist. 2016;9:79–86.
Shirley M, Scott LJ. Isavuconazole: a review in invasive aspergillosis and mucormycosis. Drugs. 2016;76:1647–57.
Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387:760–9.
Stott KE, Hope WW. Therapeutic drug monitoring for invasive mould infections and disease: pharmacokinetic and pharmacodynamic considerations. J Antimicrob Chemother. 2017;72:i12–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent
Informed consent for off-label isavuconazole administration was obtained from parents of our patient.
Additional information
Handling Editor: Vishnu Chaturvedi.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pomorska, A., Malecka, A., Jaworski, R. et al. Isavuconazole in a Successful Combination Treatment of Disseminated Mucormycosis in a Child with Acute Lymphoblastic Leukaemia and Generalized Haemochromatosis: A Case Report and Review of the Literature. Mycopathologia 184, 81–88 (2019). https://doi.org/10.1007/s11046-018-0287-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-018-0287-0