Abstract
Currently, cancer is a large contributing factor in the increased mortality rates and at present the predictions are estimating an increased trend. The conventional medical cancer imaging modalities, for example X-Ray and Computed Tomography use ionizing radiation which is not tissue friendly for repeated assessments. The Terahertz (THz) cancer imaging offers novel opportunities for non-ionizing, non-invasive and early cancer detection, or diagnosis as well as improved cancer patient treatment follow-ups. In this review, a broad overview is given on the potential of THz radiation-based imaging and sensing as a technique for detection of various cancers cells. The THz radiation dynamics and interaction mechanisms with biological systems as well as parameter extraction and modelling for the observed THz image contrast are studied. The experimental studies on THz imaging and sensing are investigated with the goal approach to investigate the ex vivo, in vitro, and in vivo observations. The use of advanced analytic algorithms, specifically deep learning, is proposed for improved detection, discrimination of complex tissue with overlapping dielectric properties and development of clinical decision support systems. Research gaps in the THz imaging studies are identified based on recent trends, latest strategies suggested and the roadmap for future research direction provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Terahertz (THz) radiation is situated in the frequency range 0.1–10 THz of the electromagnetic (EM) spectrum that is in between the Infrared and Microwave and in the transitional spectrum from electronics to photonics. THz waves are characterized by low photon energy at 3 mm to 30 μm wavelength [1]. This regime of the spectrum has been previously unexploited and known as THz gap, but with the advances in new materials, sources and detectors, the THz spectrum demonstrates potential to develop devices with sensing, detection, monitoring, imaging and spectroscopy capabilities for the healthcare application. The THz technology is also anticipated to play a crucial role in enabling the near future sixth generation (6G) wireless communication and has already been explored in the non-destructive testing (NDT), security inspection and material characterization applications [2].
The application of THz waves-based technology has attracted a vast research interest and has made remarkable progress in the biomedical application for imaging, sensing and spectroscopy due the prominent coherent detectability of the radiation, their properties of non-ionizing, non-invasive, spectral fingerprinting, and phase sensitivity to polar substances. Also, the THz waves have short wavelengths and high frequencies signal-to-noise ratio (SNR) in the time domain spectrum which enables them to carry more spectrum information. The THz waves also resonate with molecular vibrational, torsional and conformational states, enabling them to provide molecular information more than other imaging and spectroscopy technologies. Further, the THz technology is capable of reflecting more accurate differences of the targets by obtaining the pixel spectral data while acquiring THz images through the integration of THz spectra and imaging [3].
For the THz imaging measurements of tissues particularly, for the cancer detection application, the presence of tissue water molecule content has been noted to be the major source of tissue image contrast attributed to the water molecules’ high absorption coefficient and refractive index at THz frequencies. Therefore, the water molecule content differences in diseased and healthy tissues and cells causes contrast in the THz radiation responses (diseased tissue such as cancer tumours have more water molecule content relative to healthy tissue). The fluctuations of blood flow, differences in tissue structure, cell density, protein composition etc. are also significant properties for detection of cancerous tumor using THz technology [4].
The potential of THz imaging systems as diagnostic tools with capability for early detection and tissue friendly regular screening specifically for cancer demonstrates great potential to significantly curb the gradual rise of cancer related mortality rates. More advantages of THz based imaging as well as some of the limitations are outlined as follows:
1.1 Advantages of THz Imaging
-
Detection based on THz radiation is non-ionizing (since its photon energy is below ionizing radiation) and non-invasive thus it is tissue friendly for biological tissues in-vivo and ex-vivo. The technology therefore proves to be very attractive for repeated scans, treatment follow-ups as well as patient monitoring.
-
Being strongly sensitive to polar molecules like water, THz waves precisely give contrast between healthy or normal tissue and cancerous or tumorous tissue which could be a tool for early cancer tumor detection and cancer patient management.
-
The absence of Rayleigh scattering because of cell size smaller than THz wavelength provides potential for increased image resolution. Also the frequencies in the THz spectrum show an improved fatty tissue transmittance [5], thus greater resolution over Infrared based techniques.
-
THz waves are characterized by high water molecule phase sensitivity, tissue fluid composition and variable conductivity in tissue.
-
THz is very suitable for biomedical applications due to tissues from most organs having characteristic absorption in the frequency ranges within THz spectrum.
1.2 Limitations of terahertz imaging
-
THz waves are limited to macro level of the tissue images, not microscopic as in pathology.
-
There is limited penetration depth of THz signals (approximately 276 μm at 0.5THz in cancer, about 360 μm in fibrous tissues and approximately 1.142 mm in fatty tissue) because of tissue water high absorption of THz.
-
Long scanning time (due to point by point imaging), low resolution and poor SNR have also been reported to be limitations of THz imaging [6].
1.3 Related work
In this sub-section, we have comprehensively presented various reported literatures with the state-of-the-art biomedical applications particularly, the cancer cell detection. In [18], Son et al. have reviewed the biomedical THz state-of-the-art techniques, methodologies and applicable potential techniques that could revolutionize the healthcare. They surveyed some techniques for wet tissue penetration depth enhancement where they discussed methods for reaching internal organs like endoscopy and otoscopy. Further, explained the principles of operation of some THz based sensors with diabetes, breathing conditions and blood disorders sensing examples. Many of the THz biomedical applications were reported to be in cancer imaging including in detection of oral, skin, gastric, brain and breast cancers. Moreover, the potential of cancer treatment through demethylation of malignant DNA by the use of a specific high-power frequency of THz radiation are also reported with its potential as a cancer biomarker. In a similar work by Danciu [7], the detection of digestive cancers using THz based technology was reported. A summary of the THz waves characteristics, their various tissue interactions and the available THz technologies i.e., THz tomography, spectroscopy and endoscopy were reported. The review was mainly focused on reporting the research progress in THz based detection of digestive cancers – esophageal, oral, gastric, hepatic, colonic and pancreatic cancer tumors. A review of the Terahertz Pulsed Imaging (TPI), its techniques or principles, performance and applications in Biomedicine particularly in the detection of various cancers were outlined in [8].
The novel applications and future potential of THz sensing have been discussed and the optimization methods for THz data’s reflectance spectral responses in diagnosis of BCC skin cancer, colon and breast cancers described using various intelligent approaches [9]. The application of THz imaging and spectroscopy was reviewed by [10] in which both the continuous and pulsed techniques based on THz waves optical properties were used to diagnose melanoma and non-melanoma of skin tissues, assessment of scars, dysplasia and diabetes. They also highlighted the potential of THz based imaging and spectroscopy as an instrument for research and therapeutics.
In a related work by Gong et. al., [11], the applications of the biological effects of THz in biomedicine and the characterization techniques of THz in detection of cancer, protein, amino acids & polypeptides, DNA etc. were reported.
The mechanisms and biological effects of THz waves on the nervous system at the level of molecules, organisms and cells were investigated in [12]. They highlighted the future perspectives and application of THz in neuroscience and showed the nerve cell membranes, cytokines and gene expressions to be affected by THz radiation.
An overview of the state and applications of continuous wave THz (CW THz) imaging methods for biomedical samples was given in [13], with a presentation of the principle and conditions of continuous wave THz point by point scanning methods. They reported the characteristics of CW THz 3-dimensional imaging techniques, features and applications of CW THz full field imaging, they discussed CW THz imaging the biological applications. The research status, progress, advantages & limitations that hinder technological development for clinical adoption and future developments in CW THz were also summarized. This study was limited to CW THz imaging technology and its application for biomedical samples.
The potential application of THz radiation based technology as a useful tool in medicine emerging from advancements in THz technology was reviewed by [8]. They outlined the THz pulsed radiation detection techniques based on TPI and their biomedical applications. The advantages of THz pulsed radiation-based imaging were summarized, with illustration of the commercially available sources of pulsed THz radiation and corresponding coherent and incoherent detectors as well as schematic layouts for transmission and reflection TPI operating modes presented. Example application studies of TPI for in vivo and ex vivo cancer observations through the THz radiation properties were discussed. They also addressed the limitations associated with THz imaging technology for enhancement of penetration depth and sensing capabilities for biomedical applications. They noted the rapid developments in THz imaging technology and its increasing potential as a medical imaging modality, however more attention was stated to be required in the use of PEAs and more technological developments required for clinical adoption of TPI systems.
In a study reported by Wang [14], the recent developments in THz imaging technology and its application for breast tumor identification was reported. They noted the potential application of THz imaging and spectroscopy systems for breast cancer detection, with a discussion of the breast tissue dielectric properties within THz range, THz radiation sources, imaging & spectroscopy systems and THz imaging for breast cancer. The methods for improvement of data collection, processing and resolution based on chemo metrics were summarized. They also presented future research scope to address challenges in the direction of THz breast cancer imaging.
In another study, the potential biomedical application of THz technology; imaging and spectroscopy specifically for cancer, based on the features exhibited by THz radiation including low ionization energy and ability to identify biomolecules using their spectral fingerprints was reviewed [15]. They reported the recent THz imaging and spectroscopy progress in the diagnosis of cancer, with its potential to help doctors and researchers to get an insight into the cancer infected tissue area. They regarded THz spectroscopy efficient to identify biomarkers of cancer through component analysis. They also discussed the advantages and disadvantages of THz technology for cancer and auxiliary techniques for signal to noise ratio (SNR) improvement.
THz technology – THz imaging and THz spectroscopy was introduced in another study, a short overview of THz technology advances and its application for cancer diagnosis was highlighted. Being located between microwave & infrared region, THz waves are strongly sensitive to and attenuated by water through strong absorption. The characteristic properties of THz radiation such as low photon energy implying nonionizing hazard on biological tissue cause the technology to be interesting for biological applications. The image contrast between cancer and healthy tissue has been attributed to the local increase of blood supply and water content as well as the tissue structural differences [16]. More recent works have reported advanced THz imaging and sensing techniques for cancer detection including in [17,18,19]. Further, the progress in THz imaging developmental advances from 1999 to 2021 in plant, burn wound and foot diabetes imaging applications have been reported in [20] and other THz biomedical application studies particularly for cancer have been reported in [11, 13,14,15, 21,22,23].
1.4 Motivation
Cancer patients are prone to frequent & repetitive scans and therapies, with the use of conventional imaging technologies like X-Ray and CT it implies frequent exposure to ionizing radiation. For soft tissue like digestive tissue, it would not withstand the frequent clinical procedures like scans and therapies or surgeries, however the cancer related to digestive tissue have high percentage and incidence of resurfacing and increase after a treatment procedure due of unhealthy lifestyles [7]. Thus, there is a need for use of technology based on non-ionizing radiation. The repeat surgeries implies incurrence of significant emotional, temporal and fiscal costs to the patients as well as medical service providers [24], thus there is need to provide a solution that reduces those.
Technology is needed that assists surgeons with early, intra-operational and real time detection of tumorous tissue margins precisely, in order to eliminate the need for repeated surgeries when remaining malignant tissue is identified post-surgery.
Much focus in the THz imaging and sensing studies has been on the improvement of the instrumentation for example antenna [25,26,27,28,29], biosensors [19, 30,31,32,33,34] detectors [35] and probes [36]. However, less work has been done for improving the quality of the acquired image and clinical decision support improvement.
1.5 Contribution
The focus of the previously reported review work was on briefing applications of the THz imaging and sensing technology for certain cancer types and some biological applications. This work has given the comprehensive review of THz imaging and spectroscopy applications for cancer detection/ diagnosis and treatment, further:
-
Terahertz radiation dynamics and interaction mechanisms with biological systems as well as parameter extraction and modelling for observed THz image contrast are studied.
-
Experimental studies on THz imaging and sensing are investigated with an objective to investigate the ex vivo, in vitro and in vivo observations.
-
The use of advanced analytic algorithms, specifically deep learning, is proposed for improved detection, discrimination of complex tissue with overlapping dielectric properties and development of clinical decision support systems.
-
Research gaps in the THz imaging studies are identified based on recent trends, latest strategies suggested and the roadmap for future research directions are provided.
1.6 Organization
The remainder of the article is organized as shown in Fig. 1. In the second section, an overview is given of the THz imaging and sensing systems. A detailed review of THz imaging application for the detection of various cancers including the methods used is given in the third section. In the fourth section the open research challenges of THz technology are discussed and suggestions given for approaches to address the existing limitations including the proposal of a deep learning model for THz image analysis improvement. Lastly, the summary is given in the fifth section with scope for future research.
2 Overview of THz imaging, spectroscopy and sensing for cancer detection
The development of THz technology – imaging, spectroscopy and sensing has made remarkable progress and achieved significant milestones in biomedical application. Compared to conventional medical imaging technologies such as X-Ray, positron emission tomography (PET) and computed tomography (CT), the THz imaging technology offers the salient potential for label free, early cancer diagnosis and as a complementary modality to the conventional ones. Based on the type of THz radiation wave generated, the THz systems are broadly categorized as pulsed wave and continuous wave THz systems.
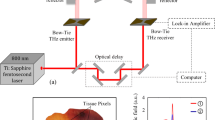
Further, the THz technology is categorized as THz imaging, THz spectroscopy and THz sensing. The development of which relies on various techniques for THz generation and detection. Some of the commonly developed THz sources are the photoconductive antennas (PCA), resonant tunneling diodes, quantum cascade lasers (QCL), Schottky diodes, gyrotrons, high speed transistors and more recently hetero-junction bipolar transistors and high electron mobility transistors show great potential. While the development of THz sources has greatly evolved, there is still need for development of more efficient, cost effective, compact and solid-state THz sources. The most common THz detectors include PCA, bolometers, Schottky diodes, optoelectronic detectors, pyro-electric detectors, Golay cell and thermocouples etc. [37]. Currently, the major THz spectroscopy systems that are being used for detection of biological samples include the photo-mixing spectroscopy, Fourier transform spectroscopy (FTS) and the THz time domain spectroscopy (THz TDS). The THz TDS (Fig. 2a) system is the most common of the THz spectroscopy systems and uses an optical source from the femtosecond laser. It mainly comprises of THz radiation generation, detection and beam guiding components. Firstly, the optical pulse is split so as to provide the detection pulse. The path length of the optical pulse is adjusted by the optical delay line and through the detection pulse’s path length change, the measurement of the test signal as a function of time is enabled.
More specifically, the principle of THz spectroscopy for identification of biomolecular samples or detection of various biological targets is based on the absorption and reflection parameter differences that are induced by the ultrafast lasers on tissue specimens. The THz spectrum systems have been used for identification of various biomolecular samples including nucleic tumor markers e.g. DNA or RNA, detection of protein tumor markers such as the CA125 and CA199 (carbohydrate antigen 125 and 199) [38] and detection of tumor cells. Through imaging, observations can be made of the morphological changes, volume, diameter etc. of the tumor which enables diagnosis and effectiveness evaluation of the treatment. The most common THz imaging system for cancer studies is the THz pulsed imaging (TPI) which can be interchangeably used with the THz TDS system. Other THz spectroscopy and imaging techniques that have been developed include the THz endoscopy, near and far field imaging, THz attenuated total reflection (THz ATR), THz tomography etc. The operational configurations of THz imaging and spectroscopy systems are the reflection, transmission and ATR depending on how the optical path of the detection pulse passes through the sample as illustrated in Fig. 2b. A detailed overview of THz instrumentation and techniques have been reported in our previous work [39].
A summary of the advantages and disadvantages of THz imaging and spectroscopy are presented in Table 1. The shortcomings of THz technology for cancer tumor diagnosis such as strong absorption of THz waves by water and size mismatch issues have facilitated the need for innovative materials and highly sensitive THz biosensing techniques. THz biosensing has been realized through the sensing techniques that include the plasmonic antennas, THz surface plasmon sensing (for example, surface plasmon polaritons (SPPs)), resonant waveguides and more recently, metamaterials (MM). MM are artificial electromagnetic subwavelength structures with potential for low cost, fast and high sensitive detection, characterized by their unique electromagnetic properties such as negative refractive index, anomalous reflection, cloaking and optical magnetism and sub-diffraction limited focusing etc. [40, 41]. Due to their compactness and small detection volume, MM show great potential for point of care diagnosis of cancer and several designs have been explored including circular ring resonators merged on a GaAs substrate and achieved a 99% absorption at 3.71THz, 1447 GHz/RIU sensitivity with 92.75 quality factor (Q-factor) [40]. More studies have explored MM based THz biosensors for early detection of various cancer markers including liver cancer [42], glioma cells [43], cervical cancer [19].
3 Application of THz imaging and spectroscopy for cancer detection
The paper emphasizes the research progress in the application of THz technology – imaging and spectroscopy envisioned as a cutting edge, near future approach for the detection of various cancers, its potential for clinical adoption and the existing challenges inhibiting its clinical environment operation. Most of the reported experimental studies on the application of THz imaging for cancer detection have been carried out on animals, human tissue and tissue phantoms.
3.1 THz image contrast mechanisms
THz image contrast is mainly attributed to the optical parameter i.e., absorption coefficient and refractive index differences owing to tissue water molecule content differences, compositional and structural changes in cancer compared to normal tissues. Pathological tissue comprise of increased water molecule content and THz radiation shows high absorption of water. Further, tissue water dielectric properties yield detectable changes in the THz range which makes water molecule content variation the most significant contrast mechanism, enabling discrimination between cancer and healthy tissues in THz imaging measurements. The dielectric response of breast tissue has been modelled at THz frequencies for distinguishing between breast tissues [44]. Biological tissues are dispersive materials and their dielectric properties such as the relative permittivity and conductivity are proportionally related to frequency, with frequency directly proportional to conductivity while inversely proportional to relative permittivity [14].
The complex permittivity of tissues can be characterized by using dielectric models to comprehend the tissues’ dielectric response for example the double – Debye model [45], single-Debye [46], Cole–Cole (CC) model [44] etc. however the Debye models can only precisely model tissue with at least 70% of water [47] and limited to low water content tissues of more complex anatomy and composition that may involve non-first order kinematics or superposition of relaxation processes [14] hence constrained to breast tissue dielectric modeling due to its heterogeneity. A mixture model comprising of a non-Debye model and single-Debye model could accurately mimic the complex permittivity spectrum of breast tissue and provide deeper, molecular level contrast mechanism [44]. For very low frequencies (below 0.1THz), the tissue complex permittivity can be modelled by a single-Debye relaxation model i.e. [44]:
where the \(\Delta {\in }_{n}\) term denotes n-th Debye relaxation process’ permittivity dispersion. For higher water content tissues e.g., skin containing about 70% water, the double Debye model comprising of two processes of Debye relation are used to model the dielectric properties of that tissue. For muscle tissue with water content higher than that of skin, a summation comprising five Debye dispersions and a frequency-based conductivity term can be used i.e.:
The low water content tissues with a complex anatomy and composition often show wider dispersion thus the molecular structure involve non first order kinematics or several relaxation processes, hence for accurately modelling the dielectric properties, the CC equation is introduced:
where \({\alpha }_{n}\) denotes distribution parameter of n-th dispersion. A Havriliak-Negami (HN) relationship introduces \(\alpha\) and \(\beta\) empirical exponents to model a non-Debye relaxation that’s constituted by non-exponential relaxation processes from the CC equation:
The mixture model comprising of Debye and non-Debye processes was reported to be best for modeling the breast tissue dielectric properties i.e.:
where, the complex permittivity’s peak real part of breast tissue is produced by the term \({\omega {\tau }_{1}\Delta \in }_{1}+\Delta {\in }_{2}\) at less than 1THz. The existence of two processes of relaxation defined by \({\tau }_{1}\) the time constant is shown through the terms \(\Delta {\in }_{1}\) and \(\Delta {\in }_{2}\). The fast relaxation mode corresponding high frequency defined by \({\tau }_{2}\) has dispersive amplitude denoted by \({\in }_{3}\) while \(\frac{\sigma }{j\omega }\) denotes dc-conductivity impact on the tissue’s dielectric loss [44].
The breast tissue has inhomogeneous anatomy and comprises fat cell and proteins where the adipose or fatty tissue has low content of water and thus contributes to the breast tissue dielectric responses. As shown in Fig. 3 reported by [14, 44], the dielectric response of breast tissues as measured by the spectrum of permittivity is high at lower frequencies and flat at high frequencies. The fibrous, fat and tumor tissues’ complex permittivity measured against the frequencies—their real and imaginary parts are shown in Fig. 3.
An average complex permittivity’s normalized percentage difference are presented in Fig. 4 for tumor, fat and fibrous tissues of the breast with the complex permittivity calculated using the measured parameters of the absorption and refractive indices.
The use of contrast-enhancing and SNR improvement agents for example Nanoparticles like the super-paramagnetic iron oxide nanoparticles (SPIO), gold nanorods (GNRs) and onion-like carbon (OLC) are also a source of contrast where the electrical interactions between the Nanoparticles and biological tissue are exploited.
3.2 THz in vitro and ex vivo imaging of cancer
In vitro and ex vivo methods based on THz technology have been mostly investigated for imaging of various cancers with the used tissue samples being excised and pretreated. The results of the THz imaging have been evaluated through comparison with histopathology results to determine the sensitivity, specificity and accuracy of THz based cancer diagnosis.
3.2.1 Breast cancer
THz imaging and spectroscopy systems have demonstrated the capability to distinguish the cancer, fibrous and adipose tissues of the breast where most of the cancers of the breast are ductal cancers and lobular cancers. This has been shown through correlations between tumor areas observed on the THz image and pathology images. However, it is still a challenging task to precisely distinguish cancer tissue and fibrous tissue. However, with advances in the development of THz systems, improved resolution has been achieved for enhanced distinction of cancer and fibrous tissues for example using THz optical fiber imaging system, THz imaging system based on Schottky diode and the use of time of flight estimation technique [3].
In a recent study [48], the breast tissue samples freshly excised from a xenograft mice model were pretreated using phosphate buffered saline solution and scanned using the pulsed THz imaging and spectroscopy system, the TPS Spectra 3000 in reflection mode. The breast tumors were cultured in the mice models using the injection of E0771 murine breast adenocarcinoma cells and excised together with adjacent fat tissues when the tumors were of 10 mm diameter when the mice are under anesthesia. The tissue specimen is placed in between two polystyrene plates that are in turn placed in the THz scanner’s scanning window and the reflection data per each pixel collected over the whole tissue surface through the set increments of the scanner motors at 200 μm step size in a fly back scanning manner. The reflected time domain pulses from each pixel are obtained and used for image reconstruction in Matlab. The magnitude variations in the collected THz pulse signal at each pixel on the tissue have been attributed to the differences in the tissue electrical property differences that in turn causes the observed tissue image contrast and distinction of the different tissue types. The image reconstruction has been performed using the Fourier Transform of the pixel-by-pixel time domain data collected. Other ways of reconstructing THz images include using the extracted absorption coefficient and refractive index for a tomographic image, time domain peak reflection image and power spectra image in frequency domain. Further, data analytic algorithms have been applied to classify the different tissue image regions of interest using fine-tuned deep learning models. In another study [14, 49], the THz TDS system has been used to scan the excised breast tissues of 2 and 10 µm thickness obtained through mastectomy.
The corresponding THz images obtained are shown in Fig. where Fig. 5a shows the magnetic and electric field-stained slide with low pathology with indications of the IDC and fibrous regions that are defined by a pathologist. Figure 5b shows the time domain-based image of de-convolved electric field while Fig. 5c and Fig. 5d present the frequency domain images at 1.5 and 1.75THz frequencies respectively. It can be observed that the THz imaging frequency domain demonstrated better discrimination of the breast tissue regions.
The design of a handheld THz pulse imaging system has been proposed for imaging of breast cancer tissue samples obtained from a breast cancer surgical procedure. When compared with the pathological tissue slices, a very high sensitivity and specificity of 87% and 96% respectively has been achieved for identifying the invasive breast cancer tissue [50].
3.2.2 Gliomas
THz technology is rapidly proving to offer cutting edge technological solutions in the intraoperative diagnosis of human brain injuries, cancer and for neuro-diagnostics. The most common brain tumor is known as gliomas. Novel studies have achieved significant progress in brain glioma THz imaging as well as distinguishing between the tissues of healthy brain tissue and glioma, providing great potential of the THz technology intraoperative brain tumor localization. THz technology has been shown to clearly differentiate margins of glioma tumor resection which is not an easy to achieve task. The image contrast between glioma and normal tissues has been attributed to increased water molecule content around the tumor region as well as cell density differences [3]. In a recent study [51], the THz TDS system has been used to explore the relationship between histological types, pathological grades, molecular pathological grades and tumor sites of freshly excised glioma specimen based on absorption coefficient which were found to be strongly correlated. The absorption spectral parameters of periphery tissue of tumor have been shown to be more distinct as compared to tumor center. Investigation of the effects of tissue samples temperature has been investigated on freshly excised samples of gliomas using THz spectroscopy where more discrimination has been shown at higher temperature based on the refractive and absorptive responses [52]. A couple of other studies have also shown THz technology’s ability to diagnose glioma of human brains, rats and mice ex vivo [53]. In a similar study by [54] a model of orthotropic glioma where ex vivo imaging of rats’ freshly excised, paraffin embedded tissue was performed in THz range, the tissue contrast of intact healthy tissue and gliomas were in both cases obtained. In freshly excised tissues, the contrast was due to increased water content because of abnormal micro vascularity, fluids around necrotic debris and edema. On the other hand, the tissue contrast for paraffin embedded tissues was due to the cell density changes in the tumor. White and grey matter contrast was observed clearly, and the contrast source was the increased myelin content in white matter. In [55] the authors used THz pulsed spectroscopy ex vivo for paraffin embedded intact tissues as well as a glioma model from mice. Contrast between tissues with tumor in paraffin embedded blocks and intact normal tissues were observed, revealing optimal spectral bands as well as features for contrast between the healthy tissues & pathological ones. A similar study [56], analyzed the tissue complex refractive index through the use of THz spectroscopy for obtaining contrast between healthy and glioma of rats with contrast mainly attributed to tumor’s increased water content. The THz pulsed spectroscopy was used to show the usefulness of THz technology in brain tumor detection. They also performed imaging of tumor with no dye using the samples obtained from a rat glioma model. They also hypothesized that complex refractive index spectrum of the THz TDS can distinguish between healthy and tumorous tissue due to higher absorption coefficient and increased values of the refractive index in cancer due to the presence of more cell density and water content relative to healthy tissues [56]. In [56,57,58] the potential of THz imaging and reflectometry was highlighted for intraoperative neuro-diagnosis using in vivo and ex-vivo models of glioma (the eGFP + + GSC-1111, U8787-MG and C66 cell lines) which were obtained from mice & rats and a few ex vivo human brain gliomas with high grade. This study mimics the human brain biophysical properties the most, while the THz dielectric response can largely differ.
In their previous work, the authors of [56] had studied the optical properties of healthy tissues embedded with gelatin and human brain glioma of WHO grades. Embedding tissue with gelatin preserved the tissues from hydration and dehydration for sustaining the tissues unaltered versus freshly excised tissues. There were statistical differences of THz response between WHO grades. The response of tissue with edema was the same as that of tumor. Thus, it was found complicated to delineate margins of edema in traumatic brain injuries and tumors.
They identified the current gap of the lack in physical models that may be used for brain tissue dielectric permittivity descriptions in the THz frequency spectrum. To bridge the gap, they used two models to analyze the THz gliomas graded as WHO Grade I-IV and healthy brain tissue by using the complex dielectric permittivity of both tissues’ ex vivo. The models used were the double over damped oscillator (DO) which uses Lorentz oscillators, the second model was the double Debye (DD) model made up of two Debye oscillators. The mathematical relation between the two models was established and they proved to accurately reproduce previous related experimental studies, where the DD models has been commonly applied in bio-photonics studies while DO satisfies the sum rule and is more rigorous. They therefore successfully applied the sum rule and the DO model for estimating water in tissues. The results prove water to be the main parameter in brain tumor tissue that causes contrast in THz. The models will play a pivotal role in advancing THz research in intraoperative neuro-diagnosis [56]. The tissue dielectric response relaxation models, the DD and DO were modelled. The relaxation terms of the DD model are as in Eq. (26) [56].
where \(\omega\) is the angular frequency = 2 \(\pi v\omega\)=2 \(\pi v\), \({\Delta }_{{\varepsilon }_{1}}, {\Delta }_{{\varepsilon }_{2}}\) are magnitudes for regulating fast and slow Debye relaxations and \({\tau }_{1}, {\tau }_{2}\) time constants for tissue dielectric response. \({\varepsilon }_{\infty }\) is the dielectric permittivity. For the DO model, two Lorentz terms as in Eq. (27) [56]:
where \({ \Delta }_{{\varepsilon }_{1}}, {\Delta }_{{\varepsilon }_{2}}, {\omega }_{01},\) \({\omega }_{02}\), \({\gamma }_{1}, {\gamma }_{2}\) are the magnitudes, quasi-resonant frequencies, damping constants respectively. Using the sum rule, water content was estimated as follows:
The imaginary part \({\varepsilon }^{\mathrm{^{\prime}}\mathrm{^{\prime}}}\) of complex dielectric permittivity in the spectral range is considered from \({\omega }_{max}\) to \({\omega }_{max}\) for tissues in numerator while denominator is for liquid water.
3.2.3 Digestive cancer
The use of THz imaging systems for digestive cancers has been investigated in experimental studies including oral, esophageal, gastric, hepatocarcinoma, colorectal, and pancreatic cancers.
Oral carcinoma:
In a study by Sim et al. [59], they assessed oral cancer using THz endoscopy. The study involved evaluating seven samples from oral cancer diagnosed patients. The authors confirmed THz endoscope as a valid tool for contrasting between normal cells and cancerous cells. They found out that the differentiation between the normal and cancerous cells was more precise and easier when frozen tissues were used for the THz imaging [59].
In another study, a bio tissue phantom has been developed in which a Bruggeman model (BM) was used to measure the optical properties of the bio tissue in THz, based on effective medium theory (EMT). Phantom development was achieved by application of graphite powders to polyvinyl chloride plastisol (PVCP). Using THz TDS in 0.2 to 0.7 THz, the phantom of 16.7% graphite and that of 31.9% were found to have optical properties correlating to oral tissue with cancer and healthy respectively [60].
Esophageal carcinoma:
An analysis of the gastrointestinal tract was done by Ji et al., [61], they used THz spectroscopy and imaging in reflection mode. Gastrointestinal tract tissue samples were obtained from a rat including the stomach, esophagus, small intestines, and large intestines with saline based perfusion while refraction of THz in water was the reference. When compared to the stomach and large intestine glandular epithelium, the absorption coefficient as well as the refractive index was found to be lesser on squamous epithelium of the esophagus. It was concluded that there is potential of increased accuracy in the application of this technique to diagnose esophagus relative to carcinomas of the intestine and gastric [61].
Gastric carcinoma:
A couple of studies have been done that used THz spectroscopy for detection of carcinoma of the gastric. In THz based measurements, the thickness of tissue contributes to the measurement accuracy, since the anatomy of gastric tissue is thick, the theoretical diagnosis of gastric carcinoma is difficult [62]. In one study 6 gastric carcinoma specimens from a surgery which were kept in NaCl 9% showed that the refraction index of gastric carcinomas and that of water are similar i.e. 2.5 whereas the refraction index of normal tissues is less than 2. The differences were suggested to be attributed to the increased blood vessels as well as edema in the tumor [62].
In another study gastric adenocarcinoma tissues obtained from surgery were prepared by paraffin embedding them and afterwards analyzed them using THz spectroscopy as well as comparing them to normal healthy tissue. Absorption peaks between 0.2 – 0.5 THz and 1 – 1.5 THz were obtained for the carcinoma. The method showed consistency with repeated experiment [63].
A similar study analyzed 21 blocks of paraffin embedded gastric adenocarcinoma at advanced stage using THz spectroscopy where the absorption coefficient and refraction index was also increased in tissues with tumor [64]. In another study [65], eight samples of gastric tissue were analyzed using THz spectroscopy where the spectral analysis showed higher refractive index values as well as higher values of absorption coefficient for the tumor tissues [65].
The performance of THz-TDS system for diagnosis of gastric tissue was investigated on surgical resected specimens in a recent study [66]. The THz absorption spectra clearly distinguished between cancer and healthy tissue with H&E staining. When compared with pathological examination results, the specificity and sensitivity were both 100% thereby demonstrating potential of THz TDS system for diagnosis gastric cancer [66].
Colorectal tumors:
THz TDS CWTI was used for assessing the absorption coefficient and refractive indices of FFPE tissue samples from human [67]. The individual sets contained a normal and 3 adenocarcinoma samples. Tumors were found to have greater absorption coefficients as well as refractive indices and it was concluded by the authors that several parameters like PH level, lipid concentration, glucose level, vascular pattern, refractive indices, absorption coefficients and cell densities have an impact on the experimental results [67]. In another study, 30 blocks of paraffin embedded colon tissue samples from human with tumor – 11pT3 adenocarcinoma & 10Pt4 adenocarcinoma and healthy were analyzed by a multipoint THz TDS in transmission mode. In their previously mentioned work, they aimed at evaluating whether cell density, abnormal vascular patterns, cell alterations or abnormal cell densities could change the contrast. The pT3 adenocarcinoma and 10pT4 adenocarcinoma were found to have an increased refractive index and absorption coefficient [68]. According to [69], absorption and reflection in THz may also be assessed without water. A study by Reid et al. [58], used THz TDS on dysplastic colon samples and normal tumor samples obtained from 30 patients showed respective 82% and 77% for sensitivity and specificity on differentiating tumor tissue from normal tissue and 89% sensitivity and 71% specificity on differentiating normal tissue from dysplastic tissue [58]. Diverse intelligent analysis methods i.e. support vector machines, decision trees and neural networks were also used in another study for re-evaluating the previously mentioned study by Reid et al., [70]. The sensitivity and specificity were increased to 90 – 100% and 86%—90% respectively thereby improving the diagnosis precision overall [70]. THz imaging based on continuous wave polarization was used for 14 fresh human colon samples of 3-5 mm thickness from 8 patients. The tissues were placed in wet compress with a pH of 7.4 and good correlation with THz imaging and histology was found [71].
In [72], differences in optical response are attributed to the differences in optical properties of healthy and cancerous colon tissues, and surface plasmon polaritons creation within their device when they proposed the use of InSb based device whose metamaterial’s spectral response changes when exposed to THz waves of the electromagnetic spectrum. Employment of THz TD—attenuated total reflection (THz TD-ATR) has been done for characterizing and differentiating lines of colon cancer cells. The spectra of the cell lines, dielectric loss tangent and absorption coefficient were measured using the Savitzky-Golay algorithm, PCA was used for feature extraction as well as cell characterization. Analysis was performed using Random Forest and the results indicated absorption coefficient as main contrast parameter for discrimination of cancer cell thus suggesting great potential for recognition of the cancer cells [73].
Hepatocarcinoma:
THz imaging has been used to analyze FFPE, formalin fixed and fresh liver tissue ex vivo. In one of the studies, the absorption coefficient for normal and hepatocellular carcinoma tissues were found to be respectively; more than 12 \({mm}^{-1}\) and from 9 – 12 \({mm}^{-1}\). The near field THz images when compared with their corresponding pathological images showed accurate cancer and normal tissue identification [74]. Since there is no standard measurement procedure, the authors proposed enhancing evaluation of tissue samples through data acquisition in mixture of paraffin and water reducing scanning time, enhance contrast and reduce background noise [75]. Hepatocellular carcinoma experimental models i.e., using rabbit and mouse models to mimic various human conditions have been developed. Through observation of the changes in polarization states of the THz wave, the baseline for healthy hepatocytes of fresh and normal tissues were established so as to develop the models [76]. In [77], the developed model was tested on liver tumor tissue of the rabbit VX2 to evaluate the model performance and the amplitudes for healthy, tumor and paraffin cells could give a distinction of healthy and cancerous regions. There was good correlation between the images and hematoxylin & eosin images with low correlation regions corresponding to regions with tumor necrosis [77]. They recommended approval of THz TDS in transmission mode for amplitude and phase pulsed signal parameter extraction and its ability to map tissue image pixel per pixel despite the long scanning time and big number of tissues samples required. They also recommended the potential use of THz digital holography for early hepatocellular carcinoma diagnosis based the variations of water content and the presence of liver fibrosis [78], THz holography was suggested to have shorter acquisition time hence faster, and could enable multi plane acquisition of information with improved structure visualization in THz frequency range [79, 80].
Pancreatic cancer:
Due to the anatomical location complexity and difficulty of accessibility of the pancreas, THz studies involving pancreatic cancer are very few. Water processed pancreatic tissue samples of 10 µm thickness were analyzed in one study [24]. Both digital slides and THz imaging were used for obtaining the images and the results showed the accuracy of THz for distinguishing between cancer cells and normal cells, also that it can assess invasiveness degree and identify 2 different tissue subtypes in the region with tumor [81].
3.2.4 Prostate cancer
The feasibility of prostate cancer diagnosis through the application of imaging based on THz radiation was examined in one study [82]. They used Terahertz Spectral Imaging (TPI) to examine paraffin embedded prostatic tissues that are partially cancerous by observing the absorption coefficient and refractive index values in normal, cancerous and prostate muscle tissues. They used three hundred and sixty absorption coefficient cases for the paraffin tissues as raw data for classification of the three tissues through Principal Component Analysis (PCA) as well as the Least Squares Support Vector Machine (LS SVM). A high accuracy of the classification was achieved and was reported to be 92.22%. Using the intensity distribution of the THz reflection signal and its absorption coefficient, they attempted to identify the tissue boundaries of the three tissues i.e., the tumor, normal and smooth muscle tissues. The three regions of interest in THz acquired images were compared with histopathological examination and the results showed a clear distinction between the tumor regions from the other tissue regions. It was concluded that TPI in conjunction with analysis based on computer mathematical technique could be efficiently used to identify prostate cancer in paraffin prostate tissue [82].
3.2.5 Cervical cancer
Tracing of living cervical cancer cells was performed in [83] using a developed THz single measurement set that uses the tilt wave front of grating pulse technique, a newly designed THz TDS technique. LiNbO3 crystal was used for generating the wave with use of a charge couple device (CCD) for detection element and enhanced radiation power from source and living cells containing water. A transient detection of the THz TD waveform of living HeLa cells was achieved with reduced scanning time in one measurement. Using Beer-Lambert law, the characteristic absorption peaks were identified at 0.49, 0.71, 1.04, 1.07, 1.26 and 1.37 THz respectively and absorbance found to be proportional to the cell concentration. Thus, detection of in-vitro cultured water containing living cervical cancer cells and living HeLa cells’ characteristic absorption spectrum in THz was realized using this system providing the potential of accurate, real-time and sensitive & insensitive e detection of human tumors based on the experimental results. In [19], an early stage identification of cervical cancer method was proposed which uses Metamaterial THz biosensor with 0.286THz and 0.850THz resonant absorption frequencies. Thus, distinguishing between cancerous and healthy cervical tissue is enabled through the sensitivity of the two resonant absorption frequency peaks to the ambient dielectric properties change.
3.2.6 Ovarian cancer
A high resolution Sub Terahertz resonance spectroscopy system was developed for biological cells and molecules together with computational analysis of molecular dynamics novel approach introduced by [84]. Optical visualization as well as quantifying the present microRNAs – specifically the mir-200 was investigated as potential biomarkers for epithelial ovarian cancer tissue samples for early detection, prognosis, analysis and treatment. The tissue samples were from archive FFPE epithelial ovarian tissue with invasive neoplastic cells regions from known papillary carcinoma cases, with normal tissues from patients without known malignancy. Using continuous wave, automated spectrometer in frequency domain of 310-500 GHz under room temperature conditions, the spectroscopic characterization of the tissue samples was performed. And the absorption spectral features whereby presence of absorption peaks close to \({13cm}^{-1}\) were identified as indicators of cancer. The diverse spectral signatures from tissue samples heterogeneity provided additional specific information that can be utilized to identify the subtypes of cancer, sensitivity to therapies and or clinical behavior. In another study, they proposed acquisition of terato-carcinoma biophysical information in real time using a resonating Plasmon obtained from meta-surface composite. For combating challenges related to skin depth of THz in bio-sensing, miniaturization with respect to frequency has been done. Programming of the Meta-material units was done so as to induce irradiative loss of energy for absorbance used for cell temperature increase targeted for ablation therapy. The resonant frequency illustrated sensitivity and power density threshold level that can be received by cells without changes in the dielectric properties determined in the far filed region experimentally [85]. Continuous wave THz spectroscopy was also found to potentially detect label free ovarian cancer in [86].
3.2.7 Lung cancer and cancer in the small intestines
In one work, THz Spectroscopic Imaging’s potential for detection of malignancies in human small intestine and lung tissues was investigated. They used the reflection mode of THz time domain spectroscopy (THz TDS) at frequency range 60 GHz – 2THz. An endoscopic sensor can reach the small intestines and lungs thus offering great potential for assessment of suspected cancerous tumors in situ. They used the reflection mode THz TDS system to characterize paraffin embedded and formalin fixed tissue blocks and confirmed that the THz responses measured shows the tissues’ property differences in electrical properties, material density and morphology. The THz band spectroscopy characteristics were contrasted with histopathologic assessment eosin and hematoxylin stained slices of tissue for the demonstration of THz spectroscopy’s potential for evaluation of small intestine and lung tissue malignancies [87].
Small intestine and lung tissue blocks that were Formalin fixed and paraffin embedded (FFPE) were obtained from the Bioserve Biotechnologies commercial tissue bank. The tissues were excised from the respiratory and gastrointestinal (GI) of 59-year-old and 62-year-old females. The standard embedding protocol for histologic diagnosis and FFPE fixation were used to prepare the tissues and pathological inspection performed. Lung tissues malignancy classification was done as well as differentiation of neural endocrine carcinoma with no necrosis. Gastrointestinal stromal tumor (GIST) characterized by diffusing to intestine wall that have necrotic parts was used to classify the malignancy of small intestines. For preparing the sample for THz spectroscopy, microtomes were used for 10 µm slices preparation for the pathologic assessment but such slices are too thin for measurements using THz spectroscopy. Post measured signal processing must also be considered to avoid etalon effects caused by the used carrier substrate. In this reported study, the samples were firstly planarized using the microtome slicing while mount on z-cut quartz window of 2 mm thickness to ensure flatness and to minimize surface roughness as well as directly exposing the actual tissue surface via the paraffin layer to enable THz reflectivity measurements. The pathological inspection processing for the last tissue slice was done through staining by Hematoxylin and eosin (H&E) thus exhibiting similar characteristics of the imaged tissue interface using reflection mode TDS and the H&E-stained microtome slice.
They used the THz TDS system—TPS3000 in reflection mode on which the tissue blocks were mounted. The THz beam was focused at 30° incident angle and ∼360 µm at 1 THz diffraction limited spot size. A resolution of 100 µm and 150 µm was used to perform raster-scanning on the lung tissues and intestine tissues respectively at ambient pressure and at room temperature. The calibration of the system was done initially through recording the reflected signal in the absence of tissue, then from there the refractive index together with the coefficient of absorption are determined. Thus, the time domain reflected signal measurement is obtained. Using the Fourier transform, \(r\) the ratio of reference electrical field responses \({E}_{r}(w,x,y)\) to the sample \({E}_{s}(w,x,y)\) is computed that is:
where the complex form refractive indices are denoted by \({\overline{n} }_{q}, {\overline{n} }_{a} and {\overline{n} }_{s}\) and the refracted angles of the air, z cut quartz and sample are denoted by \({{\theta }_{a}, \theta }_{q} and {\theta }_{s}\) respectively. \({\overline{n} }_{q}, {\overline{n} }_{a}\) and \({ \theta }_{q}\) can be determined from the known values. Using Snell’s law, \({\theta }_{a}\) and \({\theta }_{s}\) can be determined by:
where \({n}_{q}\), \({n}_{a}\) and \({n}_{s}\) are the respective quartz, air and sample refractive indices. Thus using (1) and (2), the sample’s complex refractive index and the THz properties (absorption by sample) can be extracted using:
where \({\alpha }_{s}\) and \(c\) are respectively the absorption coefficient & speed of light. The tissue sample morphological details are straight away identified using THz images that have a great correlation with the histopathological sections. Additional data for discriminating between normal tissues and pathological tissues was provided through extracted refractive indices and absorption data. Even though dehydrated FFPE tissue was used for the study, the absorption characteristics of the tissue regions of interest were observed to be the contrast source for the tissues in the THz band. The contrast can also be attributed to differences in tissue morphology, for example the different water content in hydrated tissues. They therefore concluded that there is potential to observe contrast of THz images for healthy (normal) tissues and malignant tissues both from freshly excised tissue or in situ tissues where the morphology and water content cause differences in absorption level. Also, they noted that there is a high absorptive characteristic of tissue bound with water in the THz spectrum and the parameter could be less for fresh tissues. Thus, in as much as the study initially demonstrated potential for THz imaging of organs accessible endoscopically, more studies that use fresh tissues need to be done for establishing the finding as a viable methodology for assessment of cancerous tissue margins. They also warranted use of a THz endoscopic sensor at room setting for excised tissue study.
Table 2 summarizes the most recent THz technology experimental studies in cancer applications. All the studies support the potential of THz technology as a clinical tool for cancer imaging with salient features and tissue friendliness.
4 THz in vivo imaging of cancer
The THz technology has shown significant potential for tumor localization and clear cancer tumor delineation as presented in the in vitro and in vivo cancer imaging studies previously. It is however still a hurdle to achieve in vivo imaging for most of the cancer types with the exception of cancers at the epithelial tissue layers such as skin cancer and other organs like the corneal, oral and gastrointestinal tissues. This is due to remaining challenges including low tissue penetration depth and alignment issues in THz imaging and spectroscopy measurements. For tumors of the gastrointestinal tissues i.e. hollow organs, the development of highly sensitive, flexible waveguide systems have been proposed for performing in vivo imaging [65, 97]. A good consistency of measurements has been shown for both in vivo and in vitro imaging using THz endoscopy [98] while Kaˇsalynas et al. [99], achieved in vivo imaging of the colon tissues using a novel THz imaging system with narrow band micro-bolometer sensors and differential lens.
Several studies have however demonstrated the ability of THz technology to detect various skin cancers in vivo including basal cell carcinoma, non-melanoma and melanoma skin cancers due to water molecule content differences, tissue temperature differences and nucleic acid density differences [3]. The temperature dependence property of differentiating between tissues has also been demonstrated for THz imaging of glioma in vivo using THz ATR [52]. More THz in vivo imaging studies for the cancer application have been reported in [1, 96].
4.1 Other biomedical applications of THz imaging
Several applications of THz reported including and not limited to the ones in Table 3.
5 Open research challenges and prospective opinion
As illustrated in this work, the THz technology has been investigated for cancer detection applications and exhibits the great potential for becoming a state-of-the-art medical imaging modality that complements the conventional techniques like X-Ray and MRI. The salient properties of THz radiation include low photon energy – non-invasive, non-destructive and non-ionizing, stronger penetrating ability as compared to that of the infrared radiation, biomolecular rotational and vibrational energy levels resonate at THz spectrum, the sensitivity of THz radiation to water molecules due to the presence of hydrogen bond intramolecular vibrational modes in THz is the endogenous marker for label-free tissue differentiation. The strong absorption of THz radiation by water enables tissue contrasts, more water content in tumor is attributed to the excessive metabolism in the cancer cells. However, the THz cancer cells imaging is still not yet mature and more work has to be done to further developments that are required to fit for clinical adoption.
The common limitations of THz imaging include the very high cost of commercially available THz equipment, unavailability of standardized tools and methods for enabling results comparison, high THz absorption by water and water-containing fluids under the tissue of interest causing limited penetration depth, low resolution, low signal to noise ratio (SNR), long scanning time, effects of environmental conditions on tissue samples (shape, fluid content etc.). Moreover, the current main source of tissue for most researchers is by purchasing from Biobanks, which is a very high cost for academic research. This results in limitations in the availability of data and literature in this area. So far, all the experimental data reveals that the cancer image acquisition has been performed on patches of excised or extracted tissue i.e., using invasive methods and in some cases, they used developed tissue phantoms. The THz radiation penetration depth has been observed to be mostly sufficient for carcinomas which mostly occur on the epithelial tissues non-invasively. The technology is therefore mostly suitable for the non-invasive observation of carcinomas such as of the skin. Internal epithelial tissue carcinoma like gastric, colon, mouth and stomach near the mucous membrane surfaces can also be conveniently employed through the development of miniaturized THz endoscopes. Much work has to be done so as to make the THz imaging technology non-invasive. Also, much focus of the technology improvement has been on the hardware and software improvements of the equipment, for example probes, detectors, reconstruction algorithms etc. However, there is still a limitation of the availability of efficient and cheap THz sources and detectors. THz waves also suffer atmospheric attenuation over longer transmission ranges due to water vapor as compared to Microwaves, which causes high transmission loss. THz signals are also highly vulnerable to security threats in the physical layer, causing them to be easily tapped [146].
5.1 Penetration depth enhancement: tissue preparation approaches
The limitations associated with penetration depth due to high THz absorption by water can be overcome as reported in research by using thin and/or fixed tissue samples as well as the proper geometry. The potential challenges associated with environmental changes which compromise the measurements include saline uptake, hydration level changes, humidity, scattering effects and temperature changes. For fresh tissues, when left exposed they dehydrate leading to reduced contrast features on acquisition. Thus, the methods of conserving samples are important [8]. From the reported studies, it is observed that the researchers have investigated techniques for tissue sample preparations on the excised and fixed tissue such as 1) Dehydration, 2) Alcohol perfusion, 3) Formalin fixing, 4) Gelatin embedding, 5) Lyophilizing, 6) Freezing, 7) Paraffin embedding, and 8) Paraffin emulsion.
Further, some other techniques to enhance THz penetration depth and image contrast include using contrast agents based on nanoparticles and THz penetration enhancing agents (THz PEAs) such as Glycerol has less THz absorption as compared to the water, has good tissue permeability and should be biocompatible. The development of biosensors based on antibodies and metamaterial has also been reported to improve SNR including aptamers coated with silicon dioxide layer have been proposed in [162].
5.2 Scanning time improvement techniques
Efforts are being made in the development of THz-TDS technologies to rapidly improve the rate of scanning. The THz-TDS imaging is characterized by long raster scan times caused by the single-pixel detector and mechanical linear motion of the optical delay line. Some techniques being used for scanning time improvement and to avoid optical delay line mechanical motions include the use of fast optical delay lines, cavity tuning, asynchronous optical sampling (ASOPS), electronically controlled optical sampling (ECOPS), efficient data collection methods, for example, 2D THz TDS, photoconductive antenna (PCA) integration, implementation of 2D electro-optic sampling (EOS), non-mechanical time-domain sampling and optical rectification are also useful for the acceleration of THz pulses acquisition. The advent of near field imaging and tomography in THz TDS is also significantly contributing to saving time and offering commercial potential [163]. Further, the advent of the 2D Galvano scanner, signal processing techniques or fast detector developments will improve scanning speed while real time imaging has been proposed to be achieved through a large field of view (FOV) and development of THz QCL and array of uncooled bolometer [147].
5.3 Algorithm based image improvement techniques
Conventional research on imaging technology has mainly focused on qualitative analysis of image area of interest and the use of statistical inference. The application of advanced data analytic algorithms based on machine learning such as deep learning will enable automatic quantification and ultimately achieve optimized accuracy of analysis of tissues with complexities for example tissues with dielectric overlap i.e. cancer, muscle and fiber tissues. Such algorithms also enable more precise boundary determination which is crucial in the detection of cancer tumor margins for breast conserving surgeries. Further, image processing software development for THz imaging techniques enhances image selectivity and improves the grading accuracy and analysis. The application of machine learning and deep learning techniques also helps in the development of computer-aided diagnosis (CAD) systems which aid for clinical decision support unlike the manual methods that involve time-consuming procedures, suffer interpretation variability based on subjectivities and complexity of tissues. We, therefore, propose the application of chemo metrics for the improvement of THz data analysis and image resolution through the framework of a deep learning model as shown in Fig. 6. Prior to the deep learning model for feature extraction and classification, the data goes through processing pipeline including the following stages: 1) the raw acquired THz images are input to the system, 2) preprocessing, 3) segmentation, and 4) feature extraction, 5) classification based on deep learning, and 6) the output is the results of either cancer detection or normal tissue result.
5.3.1 Preprocessing
The pre-processing of the raw materials acquired image is a crucial and initial step in the development of the CAD system employed for image quality improvement, removing unwanted noise and irrelevant background parts from the images. The pre-processing steps include the computational operations, resizing the image, RGB to Greyscale conversion, image enhancement, median filtering for noise removal, Image restoration, and Morphological operations. Previously, the histogram equalization technique has been proposed for increasing image contrast, unsharp masking Laplacian sharpening and statistical differencing for image enhancement [164].
5.3.2 Segmentation
Image segmentation is a process by which image objects or regions of interest are isolated such that the required region is obtained through partitioning, giving the object details like location and boundaries. Segmentation can be as simple as cropping out the region of interest and as complex as the application of algorithms. The algorithms used for segmentation include Region based (region growth and threshold segmentation), morphological approach, edge detection and segmentation based on clustering. Clustering based segmentation techniques include Fuzzy C-means clustering and nuclear method Fuzzy C-means clustering elaborated as follows; the Fuzzy C-means clustering (FCM) is an algorithm based on assumption that suppose a data set X of n samples i.e.\(\mathrm{X}={\mathrm{x}}_{1}\),\({\mathrm{x}}_{2}\), …..,\({\mathrm{x}}_{\mathrm{n}}\) of k data classes, cluster center\(\mathrm{C}={\mathrm{c}}_{1}, {\mathrm{c}}_{2}, \dots . , {\mathrm{c}}_{\mathrm{k}}\), with sample of the data set as \({\mathrm{x}}_{\mathrm{i}}=(\mathrm{i}=\mathrm{1,2}, \dots .,\mathrm{n})\) having cluster center point membership degree \({\mathrm{c}}_{\mathrm{j}}=(\mathrm{j}=\mathrm{1,2}, \dots .,\mathrm{k})\) where the membership degree is defined by\({\mathrm{u}}_{\mathrm{ij}}=(\mathrm{i}=\mathrm{1,2}, \dots .,\mathrm{n};\mathrm{j}=\mathrm{1,2}, \dots .,\mathrm{k})\). Then representation of the object function of the FCM Algorithm becomes:
where m is degree of ambiguity, and the value’s magnitude determines blurring degree in the clustering process. The Euclidean distance is measured by \(\Vert {\mathrm{x}}_{\mathrm{i}}-{{\mathrm{c}}_{\mathrm{j}}\Vert }^{2}\).
For the nuclear FCM method:
Considering low dimensional features \({\mathrm{R}}^{\upgamma }({\mathrm{x}}_{1},{\mathrm{x}}_{2}\in {\mathrm{R}}^{\upgamma })\), the kernel function is:
where \(\mathrm{\varnothing }()\) represents \({\mathrm{R}}^{\upgamma }\) the low dimensional space, mapping it to \({\mathrm{R}}^{\mathrm{F}}\) of higher dimensional feature i.e., the operation \(\langle \mathrm{\varnothing }\left({\mathrm{x}}_{1}\right),\mathrm{\varnothing }({\mathrm{x}}_{2})\rangle\) avoids dimensional explosion problem by mapping data to a higher dimensional space before calculating inner product. By introducing the KFCM algorithm, the objective function of the KFCM becomes:
Introducing the Gaussian kernel function to KFCM enables linear separability of the infinite dimensional space which simplifies the objective function to:
Similarly, m is the degree of ambiguity and \({\mathrm{u}}_{\mathrm{ij}}\) should satisfy three constraints. The KFCM’s objective function is based on membership constraint, extreme condition of Lagrangian is introduced to update the membership formula to:
5.3.3 Feature extraction
An image feature discriminates one part of image from another. The image features to be extracted are a reflection of the nodule pathophysiology. These are quantified using data characterization and mathematical algorithms. Various feature extraction techniques exist such as Bag of features, GLCM, PCA etc. to extract features such as color, shape, histogram and texture. The deep learning model proposed is based on the convolutional neural network (CNN) algorithm and it automatically performs feature extraction through its convolutional and pooling layers. However for the purpose of other classifiers like Support Vector Machines (SVMs), a set of feature descriptors are applied for example shape descriptors where for instance parameters can be drawn from the shape differences, morphology, texture descriptors defined by pixel by pixel parameters and lastly context relation features which can be used to indicate abnormalities based on asymmetrical measures of for instance breast e.g. Euclidean distance, eccentricity, absolute differences and Bhattacharyya distance (BD) measures etc.
5.3.4 Classification: convolutional neural network model
For automatic diagnosis of cancer, the CNN algorithm can automatically perform feature extraction from the region of interest, as well as perform classification. The CNN operations involved are summarized as Convolution operation, Max pooling, flattening and full connection. The convolution layer involves convolving the input image I with kernels or filters to produce a feature map. For a particular layer l, the produced feature map located at i, j denoted by \({h}_{ij}^{l}\), with the weight denoted by \({W}^{l}\) and bias\({b}^{l}\), the feature map is expressed as:
The Rectified Linear Unit (\(ReLu\)) is the activation function for controlling the output. For a segmented image as the CNN input, the features which characterize the segment are for example, texture and edges etc. are learned by the first convolutional layers of the CNN. More complex feature patterns are learned in the deeper layers and in the last convolution layers. To introduce non-linearity, a pooling process is performed after convolution through nonlinear down sampling. The pooling types include max, mean and stochastic pooling which helps to reduce the feature maps dimensions while getting robustness. The prediction of the required class is performed by the fully connected layers i.e., at the output. For the definition of the network, the required parameters is controlled by the network layer depth i.e., number of the layers in the network, the number of neurons in each layer and the connections of the neurons. For achieving high performance, the best weights possible are supposed to be learned during training. To enhance the classification performance and higher accuracy model, the CNN will be based on convolution and deconvolution using stacks of multiple convolution layers and multi-input CNN [148], where the number of training parameters per layer is given in (19).
where k denotes the convolution kernel scale, m is the input image channels, with n and 1 being the output images number and offset parameter, respectively. A fully connected layer is used and the PReLu as the activation function as expressed in (20) for more effective feature range between neurons.
where \({a}_{i}\) denotes the penalty factor such that when it’s equal to zero (\({a}_{i}=0\)), a ReLu function is generated. \({a}_{i}\) can be fine-tuned using a momentum function \({\Delta a}_{i}= {\mu \Delta a}_{i}+\upxi \frac{\mathrm{\delta \xi }}{\updelta {a}_{i}}\), where \(\mu\) and \(\upxi\) are the respective momentum factor and the learning rate. The optimal algorithm used for interpolation methods selection; entropy H of the gray image is computed as:
where \({p}_{i}\) denotes the pixel probability value of i-th pixel in image. For detection of cancer using Convolutional deconvolutional neural network (CDNN), a reverse reconstruction is added in each layer, reconstruction iteratively adjusted, and the feature quality is improved in forward process of the convolution. For nth convolution layer, where input is denoted as:
and output in the nth layer:
and the jth output:
where \({w}_{\left(n,j\right)}\) and \({b}_{\left(n,j\right)}\) represent the ith input & jth output of the nth convolutional layer and the activation function f(.). The formula of the activation function using PreLu is in (20).
For the inverse reconstruction (decoding) in the nth layer, let \({Z}_{n}\) be the encoding output:
where \({w}_{\left(n,j\right)}\) is the weight vector and \({a}_{\left(n,i\right)}\) is the ith input inverse. \({f}^{-1}\) is reverse activation function and \(\otimes\) the inverse convolution symbol. The loss function within each single layer is denoted by:
where \(\upgamma\) is the balance term parameter. The two terms that the loss function consists of are the reconstruction error and the regular or penalty term. The feature extraction result is denoted by:
6 Conclusion and future scope
The THz based imaging technology is increasingly becoming a novel and innovative modality for medical diagnosis of cancer which is greatly promising to significantly contribute to the evolution of healthcare systems at large. As discussed in this paper, the THz technology has been investigated for cancer cell diagnosis applications (for the types of cancer including but not limited to breast, lung, brain, digestive, skin and prostate cancers as well as great potential in cancer treatment) and shows potential as a medical imaging modality that complements the conventional techniques like X-Ray and MRI. The application of THz technology has also been reported to have great potential in applications like diabetes diagnosis, COVID-19, dental, tracing of internal scar healing and monitoring the hydration levels in-vivo. The THz technology exhibits great potential to revolutionize healthcare technology towards the cancer early diagnosis, treatment and patient care through the salient properties of THz radiation including low photon energy – non-invasive, non-destructive and nonionizing, unmatched sensing, stronger penetrating ability as compared to infrared radiation, biomolecular rotational and vibrational energy levels resonate at THz spectrum, the sensitivity of THz radiation to water molecules due to the presence of hydrogen bond intramolecular vibrational modes in THz is the endogenous marker for label-free tissue differentiation. The strong absorption of THz radiation by water is the main source of tissue contrasts, more water content in tumour is attributed to the excessive metabolism in cancer and results in optical property differences.
Although the advancement of THz based detection has been remarkable through the continuous improvement of the THz devices including miniaturization, reconstruction algorithms, sources and detectors etc., the development of the imaging technology is still not yet mature and further developments are required to fit for clinical adoption. Some of the common limitations of THz imaging include the very high cost of commercially available THz equipment, the unavailability of standardized tools and methods for enabling results comparison. THz radiation is highly absorbed by water and water-containing fluids under the tissue of interest causing limited penetration depth. Also, the technology is still associated with a lack of discriminative precision, data interpretation, data availability and analysis, low resolution, low SNR, long scanning time, and effects of environmental conditions on tissue samples (shape, fluid content etc.). Further, the reported experimental THz cancer imaging-based investigations show that the technology is mostly suitable for the observation of epithelial cancers due to the limited penetration depths.
There is still vast need to improve the THz technology through research and development for it to break into medical image modality market and achieve competitive performance to the conventional and widely used modalities like MRI, CT etc. and for the clinical adoption of THz imaging for cancer. As such, potential research challenges could be faced in addressing the aforementioned THz limitations. Some of the potential challenges are development of high-speed THz imaging systems, configurations to achieve real time, in vivo and non-invasive imaging of the body organs regardless of the location and geometry complexity, integration of the THz imaging systems with other modern technologies for example robotics, IoT etc., standardization of acquisition procedures for comparability, lack of THz image datasets for classification algorithm development. More work will be required to address the high THz absorption by water to increase penetration depth and to deliver very high SNR. Moreover, challenges related to system interoperability, standardization, security threats, cost of resources and data sharing will potentially be faced in future research.
We propose the diagnosis accuracy and analysis improvement of the acquired cancer images through development of the proposed CAD system. Future research could also investigate the integration of THz technology with robotics, internet of things, big data analytics, cloud computing etc. for remote patient monitoring, investigate THz radiation impact on biological tissues, develop cost effective, compact and sensitive THz imaging system that can be standardized for clinical trials, establish shared THz cancer image datasets to facilitate and support academic research.
References
Yin XX, Baghai-Wadji A, Zhang Y (2022) A biomedical perspective in terahertz nano-communications-a review. IEEE Sens J 22(10):9215–9227
Gezimati M, Singh G (2023) Terahertz cancer imaging and sensing: open research challenges and opportunities. Opt Quantum Electron 55(8):721–33
Zhan X, Liu Y, Chen Z, Luo J, Yang S, Yang X (2023) Revolutionary approaches for cancer diagnosis by terahertz-based spectroscopy and imaging. Talanta, pp. 124483
Gezimati M, Singh G (2023) Terahertz imaging and sensing for healthcare: current status and future perspectives. IEEE Access 11:18590
Wilmink GJ, Grundt JE (2011) Invited review article: current state of research on biological effects of terahertz radiation. J Infrared Millim Terahertz Waves 32(10):1074–1122
Cristian CR, Oliver S, Nicolae L, Vasile D, Ciortescu I, Mihai C, Stefanescu G, Emil D (2018) Advanced image processing in support of thz imaging for early detection of gastric cancer. Proc EPE 10th IntConf Exp Electr Power Eng, pp. 634–637
Danciu M, Alexa-Stratulat T, Stefanescu C, Dodi G, Tamba BI, Mihai CT, Stanciu GD, Luca A, Spiridon IA, Ungureanu LB, Ianole V, Ciortescu I, Mihai C, Stefanescu G, Chirila I, Ciobanu R, Drug VL (2019) Terahertz spectroscopy and imaging: a cutting-edge method for diagnosing digestive cancers. Materials 12(9):1519
D’arco A, Di Fabrizio M, Dolci V, Petrarca M, Lupi S (2020) Thz pulsed imaging in biomedical applications. Condensed Matter 5(2):25
Vafapour Z, Keshavarz A, Ghahraloud H (2020) The potential of terahertz sensing for cancer diagnosis. Heliyon 6(12):e05623
Nikitkina AI, Bikmulina PY, Gafarova ER, Kosheleva NV, Efremov YM, Bezrukov EA, Butnaru DV, Dolganova IN, Chernomyrdin NV, Cherkasova OP, Gavdush AA, Timashev PS (2021) Terahertz radiation and the skin: a review. J Biomed Optic 26(04):43005
Gong A, Qiu Y, Chen X, Zhao Z, Xia L, Shao Y (2020) Biomedical applications of terahertz technology. Appl Spectrosc Rev 55(5):418–438
Zhang J, Li S, Le W (2021) Advances of terahertz technology in neuroscience: current status and a future perspective. Science 24(12):103548
Zhang Y, Wang C, Huai B, Wang S, Zhang Y, Wang D, Rong L, Zheng Y (2021) Continuous-wave thz imaging for biomedical samples. Appl Sci 11(1):71
Wang L (2021) Terahertz imaging for breast cancer detection. Sensors 21(19):6465
Peng Y, Shi C, Wu X, Zhu Y, Zhuang S (2020) Terahertz imaging and spectroscopy in cancer diagnostics: a technical review. BME Frontiers 2020:2547609
Yu C, Fan S, Sun Y, Pickwell-Macpherson E (2012) The potential of terahertz imaging for cancer diagnosis: a review of investigations to date. Quant Imaging Med Surg 2(1):33–45
Sadeghi A, Naghavi SMH, Mozafari M, Afshari E (2023) Nanoscale biomaterials for terahertz imaging: a non-invasive approach for early cancer detection. Trans Oncol 27:101565
Kucheryavenko AS, Chernomyrdin NV, Gavdush AA, Alekseeva AI, Nikitin PV, Dolganova IN, Karalkin PA, Khalansky AS, Spektor IE, Skorobogatiy M (2021) Terahertz dielectric spectroscopy and solid immersion microscopy of ex vivo glioma model 101.8: brain tissue heterogeneity. Biomed Opt Express 12(8):5272–5289
Li D, Hu F, Zhang H, Chen Z, Huang G, Tang F, Lin S, Zou Y, Zhou Y (2021) Identification of early-stage cervical cancer tissue using metamaterial terahertz biosensor with two resonant absorption frequencies. IEEE J Sel Top Quantum Electron 27(4):1–7
Castro-Camus E, Koch M, Mittleman DM (2021) Recent advances in terahertz imaging: 1999 to 2021. Appl Phys B: Lasers Optics 128(1):12
Cheon H, Yang HJ, Son JH (2017) Toward clinical cancer imaging using terahertz spectroscopy. IEEE J Select Top Quantum Electron 23(4):8600109
Son J-H (2013) Terahertz molecular imaging and its biomedical applications. Terahertz Biomed Healthcare Technol, pp. SF1A.1
Lindley-Hatcher H, Stantchev RI, Chen X, Hernandez-Serrano AI, Hardwicke J, Pickwell-MacPherson E (2021) Real time thz imaging—opportunities and challenges for skin cancer detection. Appl Phys Lett 118(23):230501
Taylor ZD, Singh RS, Bennett DB, Tewari P, Kealey CP, Bajwa N, Culjat MO, Stojadinovic A, Lee H, Hubschman JP, Brown ER, Grundfest WS (2011) THz medical imaging: in vivo hydration sensing. IEEE Trans Terahertz Sci Technol 1(1):201–219
Poorgholam-Khanjari S, Zarrabi FB (2021) Reconfigurable vivaldi thz antenna based on graphene load as hyperbolic metamaterial for skin cancer spectroscopy”. Opt Commun 480:126482
Apriono C, Hidayat MV (2021) Distance investigation between two linear array microstrip antenna for terahertz breast cancer imaging. Proc AIP Conf 2344(1):50011
Kazemi F (2021) High q-factor compact and reconfigurable thz aperture antenna based on graphene loads for detecting breast cancer cells. Superlattices and Microstructures 153:106865
Samanta D, Karthikeyan MP, Banerjee A, Inokawa H (2021) Tunable graphene nanopatch antenna design for on-chip integrated terahertz detector arrays with potential application in cancer imaging. Nanomedicine 16(12):1035–1047
Yadav R, Verma A, Raghava NS (2021) A dual-band graphene-based yagi-uda antenna with evaluation of transverse magnetic mode for thz applications”. Superlattices Microstruct 154:106881
Yang K, Li J, Lamy de la Chapelle M, Huang G, Wang Y, Zhang J, Xu D, Yao J, Yang X, Fu W (2021) A terahertz metamaterial biosensor for sensitive detection of micrornas based on gold-nanoparticles and strand displacement amplification”. Biosens Bioelectron 175:112874
Azab MY, Hameed MFO, Nasr AM, Obayya SSA (2021) Highly sensitive metamaterial biosensor for cancer early detection. IEEE Sens J 21(6):7748–7755
Liu K, Zhang R, Liu Y, Chen X, Li K, Pickwell-Macpherson E (2021) Gold nanoparticle enhanced detection of egfr with a terahertz metamaterial biosensor. Biomed Opt Express 12(3):1559–1567
Zhan X, Yang S, Huang G, Yang L, Zhang Y, Tian H, Xie F, Lamy de la Chapelle M, Yang X, Fu W (2021) Streptavidin-functionalized terahertz metamaterials for attomolar exosomal microrna assay in pancreatic cancer based on duplex-specific nuclease-triggered rolling circle amplification. Biosens Bioelectron 188:113314
Lin S, Xu X, Hu F, Chen Z, Wang Y, Zhang L, Peng Z, Li D, Zeng L, Chen Y, Wang Z (2021) Using antibody modified terahertz metamaterial biosensor to detect concentration of carcinoembryonic antigen. IEEE J Select Top Quantum Elec 27(4):6900207
Habib A, Rashed ANZ, El-Hageen HM, Alatwi AM (2021) Extremely sensitive photonic crystal fiber–based cancer cell detector in the terahertz regime. Plasmonics 16(4):1297–1306
Chan KY, Ramer R (2018) Novel concept of detecting basal cell carcinoma in skin tissue using a continuous-wave millimeter-wave rectangular glass filled probe. Med Med Devices: Evid Res 11:275–285
Samanta D, Karthikeyan MP, Agarwal D, Biswas A, Acharyya A, Banerjee A (2022) Trends in terahertz biomedical applications. Generation, Detection and Processing of Terahertz Signals, pp 285–299
Lin S, Wang Y, Peng Z, Chen Z, Hu F (2022) Detection of cancer biomarkers ca125 and ca199 via terahertz metasurfaceimmunosensor. Talanta, pp. 123628
Gezimati M, Singh G (2023) Advances in terahertz technology for cancer detection applications. Optic Quantum Elec 55(2):151
Banerjee S, Dutta P, Jha AV, Appasani B, Khan MS (2022) A biomedical sensor for detection of cancer cells based on terahertz metamaterial absorber. IEEE Sensors Letters 6(6):1–4
Faruk A, Sabah C (2019) Terahertz metamaterial absorber comprised of h-shaped resonator within split-square ring and its sensory application. Optik 192:162976
Salim A, Lim S (2018) Recent advances in the metamaterial-inspired biosensors. Biosens Bioelectron 117:398–402
Zhang J, Mu N, Liu L, Xie J, Feng H, Yao J, Chen T, Zhu W (2021) Highly sensitive detection of malignant glioma cells using metamaterial-inspired thz biosensor based on electromagnetically induced transparency. Biosens Bioelectron 185:113241
Truong BCQ, Tuan HD, Fitzgerald AJ, Wallace VP, Nguyen HT (2015) A dielectric model of human breast tissue in terahertz regime. IEEE Trans Biomed Eng 62(2):699–707
Reid CB, Pickwell-Macpherson E, Laufer JG, Gibson AP, Hebden JC, Wallace VP (2010) Accuracy and resolution of thz reflection spectroscopy for medical imaging. Phys Med Biol 55(16):4825–4838
Pickwell E, Cole BE, Fitzgerald AJ, Wallace VP, Pepper M (2004) Simulation of terahertz pulse propagation in biological systems. Appl Phys Lett 84(12):2190–2192
Hurt WD (1985) Multiterm debye dispersion relations for permittivity of muscle. IEEE Trans Biomed Eng BME32(1):60–64
Liu H, Vohra N, Bailey K, El-Shenawee M, Nelson AH (2022) Deep learning classification of breast cancer tissue from terahertz imaging through wavelet synchro-squeezed transformation and transfer learning. J Infrared Millim Terahertz Waves 43(1–2):48–70
Bowman TC, El-Shenawee M, Campbell LK (2015) Terahertz imaging of excised breast tumor tissue on paraffin sections. IEEE Trans Antennas Propag 63(5):2088–2097
Grootendorst MR, Fitzgerald AJ, De Koning SGB, Santaolalla A, Portieri A, Van Hemelrijck M, Young MR, Owen J, Cariati M, Pepper M (2017) Use of a handheld terahertz pulsed imaging device to differentiate benign and malignant breast tissue. Biomed Opt Express 8(6):2932–2945
Wu X, Tao R, Zhang T, Liu X, Wang J, Zhang Z, Zhao X, Yang P (2023) Biomedical applications of terahertz spectra in clinical and molecular pathology of human glioma. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 285:121933
Wu L, Wang Y, Liao B, Zhao L, Chen K, Ge M, Li H, Chen T, Feng H, Xu D (2022) Temperature dependent terahertz spectroscopy and imaging of orthotopic brain gliomas in mouse models. Biomed Opt Express 13(1):93–104
Musina GR, Nikitin PV, Chernomyrdin NV, Dolganova IN, Gavdush AA, Komandin GA, Ponomarev DS, Potapov AA, Reshetov IV, Tuchin VV, Zaytsev KI (2020) Prospects of terahertz technology in diagnosis of human brain tumors – a review. J Biomed Photon Eng 6(2):1–11
Oh SJ, Kim S-H, Bin Ji Y, Jeong K, Park Y, Yang J, Park DW, Noh SK, Kang S-G, Huh Y-M, Son J-H, Suh J-S (2014) Study of freshly excised brain tissues using terahertz imaging. Biomed Opt Express 5(8):2837–2842
Meng K, Chen T, Chen T, Zhu L, Liu Q, Li Z, Li F, Zhong S, Li Z, Feng H, Zhao J (2014) Terahertz pulsed spectroscopy of paraffin-embedded brain glioma. J Biomed Opt 19(7):077001
Yamaguchi S, Fukushi Y, Kubota O, Itsuji T, Ouchi T, Yamamoto S (2016) Brain tumor imaging of rat fresh tissue using terahertz spectroscopy. Sci Rep 6(1):1–6
Bin Ji Y, Oh SJ, Kang SG, Heo J, Kim SH, Choi Y, Song S, Son HY, Kim SH, Lee JH, Haam SJ, Huh YM, Chang JH, Joo C, Suh JS (2016) Terahertz reflectometry imaging for low and high grade gliomas. Sci Rep 6(1):36040
Wu L, Xu D, Wang Y, Liao B, Jiang Z, Zhao L, Sun Z, Wu N, Chen T, Feng H, Yao J (2019) Study of in vivo brain glioma in a mouse model using continuous-wave terahertz reflection imaging. Biomed Opt Express 10(8):3953–3962
Sim YC, Park JY, Ahn K-M, Park C, Son J-H (2013) Terahertz imaging of excised oral cancer at frozen temperature. Biomed Opt Express 4(8):1413–1421
Zhang T, Nazarov R, Popov AP, Demchenko PS, Bykov AV, Grigorev RO, Kuzikova AV, Soboleva VY, Zykov DV, Meglinski IV, Khodzitskiy MK (2020) Development of oral cancer tissue-mimicking phantom based on polyvinyl chloride plastisol and graphite for terahertz frequencies. J Biomed Opt 25(12):123002
Bin Ji Y, Kim S-H, Jeong K, Choi Y, Son J-H, Park DW, Noh SK, Jeon T-I, Huh Y-M, Haam S, Lee SK, Oh SJ, Suh J-S (2014) Terahertz spectroscopic imaging and properties of gastrointestinal tract in a rat model. Biomed Opt Express 5(12):4162–4170
Kashanian HA, Ghaffary HB, Bagherzadeh NC (2015) Gastric cancer diagnosis using terahertz imaging. Majlesi J Multimed Process 4(4):1–7
Goryachuk A, Simonova A, Khodzitsky M, Borovkova M, Khamid A (2017) Gastrointestinal cancer diagnostics by terahertz time domain spectroscopy,” Proceedings of the IEEE International Symposium on Medical Measurements and Applications (MeMeA 2017), 2017, pp. 134–137
Hou D, Li X, Cai J, Ma Y, Kang X, Huang P, Zhang G (2014) Terahertz spectroscopic investigation of human gastric normal and tumor tissues. Phys Med Biol 59(18):5423–5440
Bin Ji Y, Park CH, Kim H, Kim S-H, Lee GM, Noh SK, Jeon T-I, Son J-H, Huh Y-M, Haam S, Oh SJ, Lee SK, Suh J-S (2015) Feasibility of terahertz reflectometry for discrimination of human early gastric cancers. Biomed Opt Express 6(4):1398–1406
Zhang Y, Han J, Wang D, Li X, Qiu T, Chen H, Chen X (2021) Application of thz time-domain spectroscopy to diagnose gastric cancer tissues in surgical resected specimens. J Infrared Millim Terahertz Waves 42(7):802–812
Wahaia F, Valusis G, Bernardo LM, Almeida A, Moreira JA, Lopes PC, MacUtkevic J, Kasalynas I, Seliuta D, Adomavicius R, Henrique R, Lopes M (2011) Detection of colon cancer by terahertz techniques. J Mol Struct 1006(1–3):77–82
Wahaia F, Kasalynas I, Seliuta D, Molis G, Urbanowicz A, Carvalho Silva CD, Carneiro F, Valusis G, Granja PL (2015) Study of paraffin-embedded colon cancer tissue using terahertz spectroscopy. J Mol Struct 1079:448–453
Wahaia F, Kasalynas I, Venckevicius R, Seliuta D, Valusis G, Urbanowicz A, Molis G, Carneiro F, Carvalho Silva CD, Granja PL (2016) Terahertz absorption and reflection imaging of carcinoma-affected colon tissues embedded in paraffin. J Mol Struct 1107:214–219
Eadie LH, Reid CB, Fitzgerald AJ, Wallace VP (2013) Optimizing multi-dimensional terahertz imaging analysis for colon cancer diagnosis. Expert Syst Appl 40(6):2043–2050
Doradla P, Alavi K, Joseph CS, Giles RH (2014) Terahertz polarization imaging for colon cancer detection. Terahertz, RF, Millimeter, Submillimeter-Wave Technol Appl VII 8985:89850K
Vafapour Z, Troy W, Rashidi A (2021) Colon cancer detection by designing and analytical evaluation of a water-based thz metamaterial perfect absorber. IEEE Sens J 21(17):19307–19313
Cao Y, Chen J, Zhang G, Fan S, Ge W, Hu W, Huang P, Hou D, Zheng S (2021) Characterization and discrimination of human colorectal cancer cells using terahertz spectroscopy. Spectrochimica Acta - Part A: Mol Biomol Spectrosc 256:119713
Chen H, Ma SH, Yan WX, Wu XM, Wang XZ (2013) The diagnosis of human liver cancer by using thz fiber-scanning near-field imaging. Chin Phys Lett 30(3):30702
Formanek F, Brun M-A, Yasuda A (2011) Contrast improvement of terahertz images of thin histopathologic sections. Biomed Opt Express 2(1):58–64
Gong Y, Dong H, Hong M, Malini O, Thong PSP, Bhuvaneswari R (2009) Polarization effect in liver tissue in terahertz band. Proceedings of the 34th International Conference on Infrared, Millimeter, and Terahertz Waves, IRMMW-THz 2009, pp. 1–2, 2009
Park JY, Choi HJ, Cho KS, Kim KR, Son JH (2011) Terahertz spectroscopic imaging of a rabbit vx2 hepatoma model. J Appl Phys 109(6):64704
Rong L, Latychevskaia T, Chen C, Wang D, Yu Z, Zhou X, Li Z, Huang H, Wang Y, Zhou Z (2015) Terahertz in-line digital holography of human hepatocellular carcinoma tissue. Sci Rep 5(1):1–6
Locatelli M, Ravaro M, Bartalini S, Consolino L, Vitiello MS, Cicchi R, Pavone F, De Natale P (2015) Real-time terahertz digital holography with a quantum cascade laser. Sci Rep 5(1):1–7
Huang H, Wang D, Li W, Rong L, Taylor ZD, Deng Q, Li B, Wang Y, Wu W, Panezai S (2017) Continuous-wave terahertz multi-plane in-line digital holography. Opt Lasers Eng 94:76–81
Brun MA, Formanek F, Yasuda A, Sekine M, Ando N, Eishii Y (2010) Terahertz imaging applied to cancer diagnosis. Phys Med Biol 55(16):4615–4623
Zhang P, Zhong S, Zhang J, Ding J, Liu Z, Huang Y, Zhou N, Nsengiyumva W, Zhang T (2020) Application of terahertz spectroscopy and imaging in the diagnosis of prostate cancer. Curr Opt Photonics 4(1):31–43
Shi W, Wang Y, Hou L, Ma C, Yang L, Dong C, Wang Z, Wang H, Guo J, Xu S, Li J (2021) Detection of living cervical cancer cells by transient terahertz spectroscopy. J Biophotonics 14(1):e202000237
Globus T, Moskaluk C, Pramoonjago P, Gelmont B, Moyer A, Bykhovski A, Ferrance J (2019) Sub-terahertz vibrational spectroscopy of ovarian cancer and normal control tissue for molecular diagnostic technology. Cancer Biomark 24(4):405–419
Saha A, Sil S, Pal S, Gupta B, Basak P (2021) Meta sensing ovarian cancer cells at thz from c band radiation biophysics. Lect Notes Netw Syst 165:111–122
Zhang J, Han Z, Yuan C, Liu S, Yao S, Song K, Su X (2020) Label-free characterization of cancerous ovarian tissues with continuous wave terahertz spectroscopy, AOPC 2020: Advanced Laser Technology and Application, 11562, 36
Yeo WG, Gurel O, Hitchcock CL, Park S, Sertel K, Nahar NK (2019) Evaluation of cancer tissue morphology via thz spectroscopic imaging: human lung and small intestine malignancies. Infrared Phys Technol 97:411–416
Vohra N, El-Shenawee M, Bailey K (2021) Terahertz imaging of enu injected sprague dawley rat breast cancer tumors. 2021 IEEE International Symposium on Antennas and Propagation and North American Radio Science Meeting, APS/URSI 2021 - Proceedings, pp. 585–586
Vohra N, Chavez T, Troncoso JR, Rajaram N, Wu J, Bailey K, El-Shenawee M (2021) Terahertz imaging of breast cancer using human and animal models,” 2021 IEEE Conf Antenna Meas Appl, CAMA 2021, 368–371
Cassar Q, Caravera S, MacGrogan G, Bücher T, Hillger P, Pffeifer U, Zimmer T, Guillet JP, Mounaix P (2021) Association of the terahertz refractive index and morphological dilation operations for breast carcinoma detection. Proceedings of the International Conference on Infrared, Millimeter, and Terahertz Waves, IRMMW-THz, vol. 2021-Augus, pp. 1–1
Chakraborty D, Mills BN, Chen G, Attanasio A, Gerber SA, Sobolewski R (2021) Pulsed terahertz time-domain spectroscopy of paraffin-embedded pancreatic ductal adenocarcinoma. Proc Int Conf Infrared, Millimeter, and Terahertz Waves, IRMMW-THz 2021:1–2
S. Yang, C. Du, L. Feng, C. Zhang, J. Wu, B. Jin, J. Chen, and P. Wu (2021) Real-time near-field terahertz spectroscopy imaging. Proceedings of the International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), 2021, 1–2
Hlali A, Zairi H (2021) Graphene based-sensor for basal cell carcinoma detection. IEEE Sens J 21(18):19930–19937
Hlali A, Oueslati A, Zairi H (2021) Numerical simulation of tunable terahertz graphene-based sensor for breast tumor detection. IEEE Sens J 21(8):9844–9851
P. Kaurav, S. Koul, and A. Basu, “Design of sub-terahertz waveguide iris probe for breast cancer tumor margin assessment,” Proceedings of the IEEE MTT-S International Microwave and RF Conference (IMARC 2021), 2021, pp. 1–3.
Yan Z, Zhu LG, Meng K, Huang W, Shi Q (2022) THz medical imaging: from in vitro to in vivo. Trends Biotechnol 40(7):816–830
Chen H, Ma S, Wu X, Yang W, Zhao T (2015) Diagnose human colonic tissues by terahertz near-field imaging. J Biomed Opt 20(3):36017
Doradla P, Alavi K, Joseph C, Giles R (2014) Single-channel prototype terahertz endoscopic system. J Biomed Opt 19(8):80501
Kašalynas I, Venckevičius R, Minkevičius L, Sešek A, Wahaia F, Tamošiūnas V, Voisiat B, Seliuta D, Valušis G, Švigelj A (2016) Spectroscopic terahertz imaging at room temperature employing microbolometer terahertz sensors and its application to the study of carcinoma tissues. Sensors 16(4):432
Taylor W, Abbasi QH, Dashtipour K, Ansari S, Shah SA, Khalid A, Imran MA (2020) A review of the state of the art in non-contact sensing for covid-19. Sensors 20(19):5665
Rao L (2020) Realization of temperature measurement by passive terahertz imaging,” Proceedings of the 13th UK-Europe-China Workshop on Millimetre-Waves and Terahertz Technologies (UCMMT 2020), 2020, pp. 1–3
Gusev SI, Guseva VA, Simonova AA, Demchenko PS, Sedykh EA, Cherkasova OP, Khodzitsky MK (2017) Application of terahertz pulsed spectroscopy for the development of non-invasive glucose measuring method,” Prog Electromagn Res Symposium, pp. 3229–3232
Hernandez-Cardoso GG, Rojas-Landeros SC, Alfaro-Gomez M, Hernandez-Serrano AI, Salas-Gutierrez I, Lemus-Bedolla E, Castillo-Guzman AR, Lopez-Lemus HL, Castro-Camus E (2017) Development of a method of evaluation of diabetic foot deterioration by terahertz spectroscopic image,” Proceedings of the International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), 2017, pp. 1–2
Hernandez-Cardoso GG, Rojas-Landeros SC, Alfaro-Gomez M, Hernandez-Serrano AI, Salas-Gutierrez I, Lemus-Bedolla E, Castillo-Guzman AR, Lopez-Lemus HL, Castro-Camus E (2017) Terahertz imaging for early screening of diabetic foot syndrome: a proof of concept. Sci Rep 7(1):42124
Hernandez-Cardoso GG, Alfaro-Gomez M, Rojas-Landeros SC, Salas-Gutierrez I, Castro-Camus E (2018) Pixel statistical analysis of diabetic vs. non-diabetic foot-sole spectral terahertz reflection images. J Infrared Millim Terahertz Waves 39(9):879–886
Boumali S, Imane B, Eddine RB, Benhabiles MT, Kerrour F (2019) Design of a cost-efficient terahertz system for biomedical applications. Proc Adv Sci Eng Technol Int Conf (ASET 2019), 2019, pp. 1–5
Smolyanskaya O, Petrov N, Nazarov M, Vaks V, Kistenev Y, Cherkasova O, Guillet JP, Mounaix P, Shkurinov A (2020) THz spectroscopy, holographic approach and phantoms design for the diagnostics of socially significant diseases,” Proc Int Conf Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), 2020, pp. 354–355
Lykina A, Konnikova M, Chernyaeva M, Gavrilova P, Mustafin I, Domracheva E, Anfertev V, Vrazhnov D, Prischepa V, Toropova Y, Korolev D, Shkurinov A (2020) Study of dry pellets of blood plasma using thz spectroscopy,” Proceedings of the International Conference Laser Optics (ICLO 2020), 2020, 1–2
Fan S, Ung BSY, Li CT, Pickwell-MacPherson E (2015) Terahertz reflection imaging of hypertrophic scar tissue in vivo. Proceedings of the 40th International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz 2015), 2015, pp. 1–2
Huang SY, Wang YXJ, Yeung DKW, Ahuja AT, Zhang YT, Pickwell-Macpherson E (2009) Tissue characterization using terahertz pulsed imaging in reflection geometry. Phys Med Biol 54(1):149–160
Taylor ZD, Singh RS, Culjat MO, Suen JY, Grundfest WS, Lee H, Brown ER (2008) Reflective terahertz imaging of porcine skin burns. Opt Lett 33(11):1258–1260
Fan S, Ung BSY, Parrott EPJ, Wallace VP, Pickwell-MacPherson E (2017) In vivo terahertz reflection imaging of human scars during and after the healing process. J Biophotonics 10(9):1143–1151
Ke L, Wu QYS, Zhang N, Liu HW, Teo EPW, Mehta JS, Liu YC (2021) Ex vivo sensing and imaging of corneal scar tissues using terahertz time domain spectroscopy”. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy 255, 119667
Wang J, Sun Q, Stantchev RI, Chiu T-W, Ahuja AT, Pickwell-MacPherson E (2019) In vivo terahertz imaging to evaluate scar treatment strategies: silicone gel sheeting. Biomed Opt Express 10(7):3584–3590
Shi J, Wang Y, Chen T, Xu D, Zhao H, Chen L, Yan C, Tang L, He Y, Feng H, Yao J (2018) Automatic evaluation of traumatic brain injury based on terahertz imaging with machine learning. Opt Express 26(5):6371–6381
Hua Y, Zhang H (2010) Qualitative and quantitative detection of pesticides with terahertz time-domain spectroscopy. IEEE Trans Microw Theory Techniq, 58(7 PART 2):2064–2070
Yum SM, Baek IK, Hong D, Kim S, Eom K, Jang J, Kim S, Sattorov M, Lee MG, Kim S, Adams M, Park GS (2019) Controlled hydration in epidermal ridges probed by thz time-domain spectroscopy,” Proceedings of the International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), 2019, pp. 1–2
Suen JY, Tewari P, Taylor ZD, Grundfest WS, Lee H, Brown ER, Culjat MO, Singha RS (2009) Towards medical terahertz sensing of skin hydration. Stud Health Technol Inform 142:364–368
Bennett DB, Li W, Taylor ZD, Grundfest WS, Brown ER (2011) Stratified media model for terahertz reflectometry of the skin. IEEE Sens J 11(5):1253–1262
Alfaro-Gomez M, Ramos-Soto D, Saucedo E, Singh Ak, Castro-Camus E (2019) Terahertz imaging of moisturizer interaction with the skin in-vitro,” Proceedings of the International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), 2019, pp. 1–2
Horita K, Akiyama K, Sakamoto T, Takahashi K, Satozono H (2020) Terahertz time-domain attenuated total reflection spectroscopy using flow-through method for continuous analysis of dehydration process, Proceedings of the International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), 2020, 61–62
Lopes DS, Souza WS, de Araujo RE, Gomes ASL (2019) Terahertz time-domain spectroscopy of healthy and carious dental tissues,” Optics InfoBase Conference Papers, Part F140, 29
Kamburoglu K, Yetimôglu NO, Altan H (2014) Characterization of primary and permanent teeth using terahertz spectroscopy. Dentomaxillofacial Radiology 43(6):20130404
Sim YC, Maeng I, Son JH (2009) Frequency-dependent characteristics of terahertz radiation on the enamel and dentin of human tooth. Curr Appl Phys 9(5):946–949
Amaechi BT (2009) Emerging technologies for diagnosis of dental caries: the road so far. J Appl Phys 105(10):102047
Fedorov VI (2016) The study of terahertz radiation biologic effects as premise for creating of diagnostic and treatment methods, Proc 2016 Int Conf Laser Optic, 2016, S212
Bagraev NT, Klyachkin LE, Malyarenko AM, Novikov BA, Presnukhina AP, Reukov AS, Taranets KB, Khromov VS (2020) Prospects for the use of silicon terahertz sources for the treatment of pulmonary pathologies and affections caused by covid-19,” Proceedings of the International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), 2020, 837–838
Son JH, Cheon H (2018) Toward cancer treatment using terahertz radiation: demethylation of cancer dna, Proceedings of the International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), 2018, pp. 1139002
Seki T, Ohki Y, Mizuno M (2020) Terahertz and far-infrared absorption spectroscopic study of dna bases,” Annual Report - Conference on Electrical Insulation and Dielectric Phenomena, 2020, pp. 539–542
Singh AK, Wen C, Vinh NQ (2020) Hydration dynamics in deoxyribonucleic acid using an extended mhz-thz spectroscopy,” Proceedings of the International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), 2020, pp. 762–763
Weisenstein C, Schmeck M, Schaar D, Wigger AK, Bosserhoff AK, Bolivar PH (2019) Quantification of dsdna functionalization efficiency in thz biosensors. Proceedings of the International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), 2019, 1–2
Wang H, Yang Z, Li D, Geng G, Li Z, Yan S, Cui HL (2019) Imaging biological samples using far-and near-filed thz microscopy, Proceedings of the International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), 2019, 1–3
Cheon H, Yang HJ, Lee SH, Kim YA, Son JH (2016) Terahertz molecular resonance of cancer dna. Sci Rep 6:1–10
Semenova AV, Choporova YY, Cherkasova OP, Vaks VL, Smolyanskaya OA (2017) Experimental and theoretical study of dna gyrotropy at thz frequencies. Proceedings of the International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), 2017, pp. 1–2
Yan S, Xia L, Wei D, Cui HL, Du C (2017) Terahertz biosensing of protein based on a metamaterial. IEEE 3M-NANO 2016 - 6th Proceedings of the IEEE International Conference on Manipulation, Manufacturing and Measurement on the Nanoscale, pp. 327–330
Liu Y, Wang F, Zhao D, Ling J, Li S (2016) Study on thz spectroscopic characteristic of four dna nucleobases. Proc Int Conf Infr, Millimeter, Terahertz Waves, IRMMW-THz 2016:1–2
Vaks VL, Semenova AV, Khodzitsky MK, Odlyanitskiy EL, Sedykh EA, Balya VK, Smolyanskaya OA (2016) Gyrotropy and absorption of dna and amylose at thz frequencies. Proc Int Conf Infr, Millimeter, Terahertz Waves, IRMMW-THz 2016:1–2
Bagraev NT, Chernev AL, Klyachkin LE, Malyarenko AM, Emel’Yanov AK, Dubina MV (2016) DNA detection by thz pumping. Proceedings of the International Conference on Infrared, Millimeter, and Terahertz Waves, IRMMW-THz, 2016-Novem(7):944–948
Wang F, Li M, Jiang L, Liu Y (2016) Terahertz spectral signature of dna macromolecules, Proceedings of the 2015 Asia-Pacific Microwave Conference (APMC), 2, 1–3
Duka M, Balbekin N, Smolyanskaya O, Sobakinskaya E, Panin A, Vaks V (2015) Characterization of dna absorption lines in the terahertz range. Proceedings of the 2015 IEEE North West Russia Section Young Researchers in Electrical and Electronic Engineering Conference ElConRusNW, 2015, 327–328
Konnikova M, Cherkasova O, Nazarov M, Vrazhnov D, Kistenev Y, Shkurinov A (2020) Terahertz spectroscopy of blood plasma as a promising method for diagnosing of thyroid cancer. Proc Int Conf Infr Millimet Terahertz Waves, IRMMW-THz 2020:846–847
Hakeem SI, Hassoun ZA (2020) Skin cancer detection based on terahertz images by using gabor filter and artificial neural network. IOP Conf Series: Mat Sci Eng 928(3):32025
Boutaayamou M, Cerica D, Verly JG (2020) Terahertz pulsed imaging of dehydrated human breast cancer samples. Proceedings of the International Conference on Infrared, Millimeter, and Terahertz Waves, IRMMW-THz, 2020-Novem, pp. 65–66
Serita K, Okada K, Tonouchi M (2020) Scanning point terahertz source microscope and terahertz microfluidic chip for biological applications. Proc 2020 Int Topic Meet Microw Photon, MWP 2020 - Proceedings, pp. 108–111
Konnikova MR, Cherkasova OP, Nazarov MM, Vrazhnov DA, Kistenev YV, Titov SE, Kopeikina EV, Shevchenko SP, Shkurinov AP (2021) Malignant and benign thyroid nodule differentiation through the analysis of blood plasma with terahertz spectroscopy. Biomed Opt Express 12(2):1020–1035
Bharadwaj AN, Kashyap AM, Jayachandran R, Kishore R (2023) A survey on terahertz devices-a cutting edge technology. 2023 Proceedings of the International Conference on Recent Trends in Electronics and Communication (ICRTEC), pp. 1–6
Son JH, Oh SJ, Cheon H (2019) Potential clinical applications of terahertz radiation. J Appl Phys 125(19):190901
Wang Y, Yang F, Zhang J, Wang H, Yue X, Liu S (2021) Application of artificial intelligence based on deep learning in breast cancer screening and imaging diagnosis. Neural Comput Appl 33(15):9637–9647
Funding
Open access funding provided by University of Johannesburg.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors claim no conflicts of interest. This is the author’s declaration that guarantees objective and fair research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gezimati, M., Singh, G. Terahertz imaging technology for localization of cancer tumours: a technical review. Multimed Tools Appl 83, 33675–33711 (2024). https://doi.org/10.1007/s11042-023-16596-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11042-023-16596-z