Abstract
Left subclavian artery occlusion is frequently treated by stent placement. The most commented on In-Stent Restenosis is excessive thrombus that squeezes the stent and leads to vessel occlusion. In this paper, an automatic system is provided to segment the left subclavian arteries and left aortic arches from chest MRI (Magnetic Resonance Imaging) images, and then to identify the occlusion of left subclavian artery based on the gray-levels of the extracted left subclavian artery and left aortic arch. Experimental results show that the system obtains the accuracy rate 97.33% of detecting the occlusion of left subclavian artery. The other task in this paper is to explore the relationship between stent/vascular diameter ratio and restenosis of left subclavian artery after stenting. Besides that, the experimental results also show that Assurance is useful for reducing the restenosis of left subclavian artery with stent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
According to statistics from Taiwan’s Ministry of Health and Welfare in 2018, heart disease and cerebrovascular disease ranked second and fourth in Taiwan, indicating that cardiovascular disease has seriously endangered people’s health. Vascular disease is often caused by high blood pressure and high blood sugar, which may be related to dietary habits, obesity, smoking, diabetes, and family history [44]. When there is too much cholesterol in blood, fat will accumulate on vascular wall, which may cause various vascular diseases, such as atherosclerosis, heart disease, hypertension, stroke, and myocardial infarction [18].
The cardiovascular circulatory system uses blood circulation to supply blood to organs throughout the body. The aorta is the main trunk of the arterial system. Arteries with abundant elastic fibers closed to the heart maintain the pressure gradient. When the left ventricle contracts, blood is squeezed out which conducts blood to smaller branches. Ascending aorta and aortic arch, and descending aorta are the extensions of aorta. From right to left, the head wall artery (Brachiocephalic trunk), the common carotid artery, and the subclavian artery are the three branches of the aorta emerging from the convex side of the aortic arch.
The subclavian artery is to transport blood to head and arm [5]. When blood is insufficiently supplied to the ipsilateral vertebral artery, the patient may have the following symptoms: light-headedness, confusion, dizziness, motor deficits, visual disturbances, focal seizures, vertigo, and ataxia. Subclavian artery stenosis may cause upper-extremity claudication and fatigue, even incite angina pectoris due to coronary artery steal with retrograde blood flow occurring when performing an upper-extremity exercise [31]. Common symptoms of subclavian artery stenosis are bruits over the subclavian artery, blood pressure decrease, and intermittent claudication of the arm on the same side as the subclavian artery. The left subclavian artery is more often obstructed than the right subclavian artery. Figure 1 demonstrates an In-Stent no Restenosis image and an In-Stent Restenosis image. The main purpose of this paper is to develop an automatic system to identify the occlusion of left subclavian artery from a chest MRI image.
Mild subclavian artery stenosis can be improved through drug and healthy dietary habits. Severe subclavian artery stenosis is often treated by vascular bypass graft, balloon angioplasty, or stenting surgery. Restenosis rates are quite high in patients after stenting. Vascular stents are widely employed in the treatment of the arterial stenosis [3]. Vascular stent, a special cylindrical metal net with a balloon catheter, is inserted into a narrow vascular. The balloon is inflated and then removed, and the stent is left in in the vascular to keep the artery open. However, the implantation of stents in arteries will trigger platelet activation, which may cause thrombus formation and subsequent Stent Thrombosis.
Stent thrombosis is an acute process which is a gradual narrowing of the stent lumen due to neointimal proliferation. Over a half of patients after stenting occur moderate restenosis. For the patients with severe occlusion of left subclavian artery, heart stent surgery is a common treatment option; however, 15% to 20% of the patients after stent surgery occurred In-Stent Restenosis (Fig. 1) [3]. Excessive thrombus is the leading cause of In-stent restenosis. Drug-eluting stents can attenuate neointimal formation, and result in a reduction of the incidence of In-Stent Restenosis to rates ranging from 5% to 10%. Although Drug-Eluting Stent can reduce this chance, they still cannot completely avoid In-Stent Restenosis [35].
Stent, an expandable device, is fed into the artery via a balloon catheter to keep the artery open. Each stent has the most suitable extension size. If the stent is expanded too much, some problems may arise, such as, stent deformation or fracture, which will make its supporting capacity decreasing [29]. It is important for which type and size of stent should be for most effective at preventing from restenosis after surgery.
Artificial intelligence technologies can complement the knowledge of doctors. Diagnostic imaging plays a critical role in accurately screening, diagnosing, and planning effective management for atherosclerotic cardiovascular disease [20]. Y. Horiuchi et al. [21] partition the acute heart patients into 3 distinct clusters via artificial intelligence technology according to the symptoms and characteristics of the patients. The clusters also achieved the validation of clinical results. In [31], a semi-automated software based on plaque characterization was employed to predict subsequent cardiac death during long-term follow-up. M. Zreik et al. [48] integrated convolutional neural network [16], unsupervised convolutional autoencoder [37], and SVM [11] to identify the patients with a functionally significant coronary artery stenosis. M. C. Lancaster et al. [26] measured the left ventricular diastolic dysfunction for isolating high-risk phenotypic patterns and assessing their prognostic significance based the natural clustering of echocardiographic variables. A fully automatic neural network regression-based algorithm in [39] was developed to segment the left ventricle from cardiac MRI for improving the efficiency and reproducibility of clinical assessment.
Image recognition techniques describe and identify the characteristic of medical images. Image recognition techniques have hence become important tools for image processing experts and clinicians to establish intelligent precision medicine [14]. Noninvasive imaging plays an important role in the management of patients with cardiovascular disease. Subjective visual interpretation has the clinical mainstay and quantitative analysis facilitates objective, evidence-based management. It has driven the developments in computing and software tools aimed at achieving fully automated image processing and quantitative analysis [36].
Ultrasound imaging can quantify carotid arterial wall structure to assess the cerebral and cardiovascular disease risks [9]. A. T. Papageorghiou et al. [1] quantified cardiac morphology and function, and created computational models to identify geometric changes based on Cardiac ultrasound. M. Sawicki et al. [34] investigated the accuracy of AI-based software for detecting large vessel occlusion with computed tomography angiography in stroke patients using an experienced neuroradiologist’s evaluation as the reference. In [25], an automatic system is provided to detect, grade, and classify coronary stenosis in coronary computed tomography angiography caused by all types of plaques. J. H. Baek et al. [2] indicated that occlusion type identified with computed tomography angiography could predict endovascular treatment success via stent retriever thrombectomy. M. Meijs et al. [28] presented a convolutional neural network for image-level detection of intracranial anterior circulation artery occlusions in 4D computed tomography angiography.
Despite that many stents are implanted every year worldwide, in-stent restenosis is the most common complication and still poses a significant risk to patients. X. Chen et al. [6] developed a stent equipment with microscale sensors and wireless interface to continuously monitor the restenosis through the implanted stent. This device can wirelessly detect the blood clot formation in a swine model. Chowdhury et al. [8] applied Vascular Positron Emission Tomography to predict Restenosis following lower limb percutaneous transluminal angioplasty.
The preferred route is from the left subclavian arteries to the aorta for many requiring upper extremity access treatments. However, it is often encountered asymptomatic left subclavian artery occlusion [30]. Subclavian artery thrombosis is generally felt to be underdiagnosed, which will cause obstruction of blood flow through the subclavian artery. The thrombosis incidence of the left subclavian artery is four times higher than that of the right subclavian artery. It will cause severe ischemia in the brain, upper extremities, and occasionally the heart [31]. However, there is no good way to predict restenosis of left subclavian arteries after stenting.
This research hence intends to develop a system to automatically detect whether a left subclavian artery with stent is obstructed based on the chest MRI image. We call the system left subclavian artery obstruction (LASAO) detector. The LASAO detector extracts the aortic arch and left subclavian artery areas from a chest MRI image, and then identifies the occlusion of left subclavian artery. There are many noise objects, such as aortic arch, left common carotid artery, brachiocephalic artery, thoracic spine, sternum, stent, and so on, which make the left subclavian artery and aortic arch extracted from the stent-LSA region difficult, and the size of left subclavian artery is very small. The LASAO detector applies the color, spatial, temporal, and shape features to isolate the left subclavian artery and aortic arch from the stent-LSA region from a chest MRI image. The LASAO detector is helpful for the medical professional to detect whether a left subclavian artery with stent is obstruct.
Moreover, in this research, which size of the stent should be for the patient with obstructive left subclavian artery is also been statistically analyzed. It is useful for the medical professional to select the most suitable stent so that the incidence rate of In-Stent Restenosis can be reduced after vascular stent surgery. In this research, we also investigate the efficacy of the stent with assurance in preventing from In-Stent Restenosis.
2 The LASAO detector
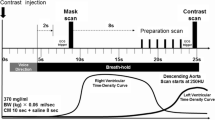
A fluorescein angiography is to inject a fluorescent dye into the bloodstream, so that the blood flow in vessel can be observed by imaging the fluorescence. Generally, the healthy aortic arch and the healthy left subclavian artery are bright since a fluorescent injected into veins makes the veins in a chest MRI image clearer. The healthy aortic arch is much brighter than the obstructive left subclavian artery. Let gLAA be the average gray-level of the pixels in the left aortic arch (LAA) and gLSA the average gray-level of the pixels in the left subclavian artery (LSA). The LASAO detector considers the left subclavian artery is occluded if
where Tg is a given constant. The LASAO detector hence first segments the left subclavian artery and the left aortic arch from a chest MRI image; then it identifies the obstructive left subclavian artery based on gLAA and gLSA.
Therefore, the following of this paper will describe the two phases in details: stent-LSA object segmentation and SLAA object segmentation. The goal of the stent-LSA segmentation phase is to extract the left subclavian artery from the chest MRI images. A stent is embedded into the left subclavian artery. Hence the stent and the left subclavian artery will be combined into one; we name it a stent-LSA object. The LASAO detector first extracts the stent-LSA object from a chest MRI image and then separates the LSA object from the stent-LSA object. The SLAA object segmentation phase is to extract the stent with left aortic arch (SLAA) object. It then extracts the aortic arch from the SLAA object. Figure 2 displays the framework of the LASAO detector.
2.1 Stent-LSA object segmentation
This subsection is to describe the phrase of stent-LSA object segmentation in details. This phrase is divided into three stages: stent-LSA object locating, stent-LSA object isolation, and LSA object segmentation. The stent-LSA object locating stage is to stretch the contrast of an original chest MRI image and locate the stent-LSA object in chest MRI image for the subsequent LSA object segmentation. The stent-LSA object isolation stage is to remove noise that cannot be the stent-LSA object. The LSA object segmentation stage is to extract the left subclavian artery from stent-LSA object.
2.1.1 Stent-LSA object locating
In this paper, 37 chest cross sectional anatomy MRI images, taken from each of 46 patients wearing stent on their left subclavian artery, are used as test samples which are provided by China Medical University Hospital, Taichung, Taiwan. All the chest cross sectional anatomy MRI images, each of which comprises 512 × 512 pixels, are grouped into a data set SD; therefore, SD consists of 1702 chest cross sectional anatomy MRI images. Here, we call a chest cross sectional anatomy MRI image a chest MRI image. The chest MRI images may contain tissues, blood vessels, bones, stents, and so on. As shown in Fig. 3a, the stent-LSA object occupies only a very small part of a chest MRI image. Since the left subclavian artery is located on the left side of the chest and spine, the LASAO detector first locates the approximate position of the stent-LSA object on a chest MRI image, as shown the red rectangle in Fig. 3b.
The LASAO detector first employs gamma correction operation [17, 46] to stretch the gray levels of the chest MRI image I0. Let I0(x, y) be the gray level of the pixel located at the coordinates (x, y) on I0; also let Min0 and Max0 be the minimal and maximal gray levels of the pixels on I0. Gamma correction operation will convert I0 into Ig by the following formula:
where r0 is a given constant.
The LASAO detector then transforms Ig into a binary image IO via Otsu’s thresholding operation [32]. Otsu’s thresholding operation can be defined as
where.
- ||Ig||:
-
the total number of the pixels in Ig.
- N1:
-
the total number of the pixels in Ig with the gray levels less than or equal to Ti.
- N2:
-
the total number of the pixels in Ig with the gray levels larger than Ti.
- V 1 ( T i ) :
-
the gray level variation of the pixels in Ig with the gray levels less than or equal to Ti.
- V 2 ( T i ) :
-
the gray level variation of the pixels in Ig with the gray larger than Ti.
Generally, vessels and stent are brighter since a fluorescent is injected into veins to make the veins in a chest MRI image clearer. If I0(x, y) ≤ TO, the LASAO detector sets Ig(x, y) to 0, and else sets Ig(x, y) to 1. After that, the LASAO detector only keeps the biggest white object and removes other white objects. The remained white object is the chest. Figure 4a illustrates the obtained image after running gamma correction operation on the image in Fig. 3a. Figure 4b is the result obtained by running Otsu’s thresholding operation on the image in Fig. 4a and only the biggest white region is remained.
The result image obtained by running Gamma correction and Otsu’s thresholding operations on image I0 in Fig. 2a
The LASAO detector next extracts the region in which the stent-LSA object approximately locates, we call it the stent-LSA region. The left aortic arch and the left subclavian artery with stent are located at the left side of the chest object. The LASAO detector calculates the gravity center G(\( \overline{\boldsymbol{x}},\overline{\boldsymbol{y}} \)) of the chest object by the formula:
where nc is the number of pixels in the chest object. The LASAO detector then sets the stent-LSA region Ra with the upper left corner (\( \overline{\boldsymbol{x}}-{\boldsymbol{l}}_{\boldsymbol{x}\boldsymbol{l}},\overline{\boldsymbol{y}}-{\boldsymbol{l}}_{\boldsymbol{y}\boldsymbol{u}} \)) and the lower right corner \( \left(\overline{\boldsymbol{x}}+{\boldsymbol{l}}_{\boldsymbol{x}\boldsymbol{r}},\overline{\boldsymbol{y}}+{\boldsymbol{l}}_{\boldsymbol{y}\boldsymbol{d}}\right) \). In Fig. 5a, the red dot is the gravity center (\( \overline{\boldsymbol{x}},\overline{\boldsymbol{y}} \)) of the chest object, and the red region is the approximate stent-LSA region. Figure 5b shows the segmented approximate stent-LSA region Ra.
2.1.2 Stent-LSA object isolation
MRI (Magnetic Resonance Imaging) is a scanning technique for generating detailed images of parts of human body. The scanning technique employs a strong magnetic field and radio waves to create the images of parts of human body. When radio waves are sent to the body from different directions, MRI may obtain different tomographic images. Hence, the shapes of the stent-LSA object in a set of MRI images may be different that will make the segmenting the stent-LSA object from the stent-LSA region difficult. Additionally, a stent-LSA region may contain noise objects, such as Aortic arch, left common carotid artery, brachiocephalic artery, thoracic spine, sternum and so on, which will be removed from the stent-LSA region.
-
A.
Spine and Sternum Objects Removing
This step is to remove the spine and sternum objects from the stent-LSA region. Spine and sternum are brighter objects and located at the top and bottom of the stent-LSA region. Otsu thresholding method [32] is a thresholding method depending only on the gray-levels of an image. The LASAO detector converts the gray-level stent-LSA region Ra into a binary region Rb based on the threshold To obtained by Otsu thresholding method from the gray-levels of the pixels in Ra. If Ra(x, y) ≥ To, then the LASAO detector assigns ‘1’ to Rb(x, y); otherwise gives ‘0’ to Rb(x, y). The top and the bottom bright objects in Rb are respectively the spine and sternum objects. The LASAO detector hence removes them. Figure 6a shows the processing of removing the spine and sternum. After that, the opening operation [10] with the structure element shown in Fig. 6b is used to eliminate the small bright spots in Rb.
-
B.
Non-Stented Objects Removing
After removing the spine and sternum objects, there are still some non-stented objects in Rb. This step intends to remove other non-stented objects in Rb. In this step, for each object in Rb, the LASAO detector employs convex hull operation [23] to draw the smallest convex polygon which contains the whole object. The object with a small convex polygon or a long striped convex hull is considered to a non-stented object; hence, the LASAO detector gets rid of the object from Rb. Figure 7b displays the convex hulls of the objects in Fig. 7a. Let Dl be the longest distance between two pixels on the convex hull of an object and Al the area of the convex hull of the object. The shape of the convex hull can be described as follows:
Sl is closed to 0 for a long striped object while Sl is near to 1 for a short rounded object. Hence, when Sl < Ts or Al < Ta, the object is removed from Rb, such as Fig. 7c. Let Rl be the region with the remaining objects, such as Fig. 7d.
-
C.
Stent-LSA Object Extracting
Rl may still contain some noise. The goal of this step is to extract the stent-LAS object from Rl. Blood vessels and stents are three-dimensional shapes; therefore, the same blood vessel or stent will appear to multiple continuous MRI slices. The LASAO detector hence regards an object as a stent-LAS object if it also appears in previous or next chest MRI image. It means that if an object in Rl is overlapped with one object in previous image or next image, then the object is considered to a stent-LAS object, for the example shown in Fig. 8. Let Rsl be the result region obtained by this step.
2.1.3 LSA object segmentation
A stent is embedded into the left subclavian artery. This stage is to separate the left subclavian artery (LSA) from the stent-LAS object in Rsl. The contour of the left subclavian artery is a closed curve. The expected thickness of an object contour is one-pixel. In this stage, the lines in Rsl are attenuated to one-pixel thickness. Broken contour lines may occur due to low quality of MRI images. The LASAO detector then connects the discontinuing object contour. Moreover, it finally decides the LSA object in Rl via the overlap with one object in previous image or next image.
For each pixel Rsl(x, y) in Rsl, the thinning operation [10] gives a window Wt(x, y) which consists of 3 × 3 pixels in Rsl and Rsl(x, y) is the center of the window Wt(x, y). The thinning operation compares Wt(x, y) with each of the eight structuring elements shown in Fig. 9, the gray pixels in which is “don’t care bits” (it does not matter whether they are ‘0-’ or ‘1-’ bits). We call Wt(x, y) is matched if the values and the positions of 0- and 1-bits in one of the eight structure elements are completely the same as the pixels on Wt(x, y), regardless of don’t-care bits. If Wt(x, y) is matched, Rsl(x, y) = 0. The thinning operation repeatedly runs the procedure mentioned above, until no pixel can be changed. Let Rt be the result obtained by running the thinning operation on Rsl. Figure 10b demonstrates the result obtained by performing the thinning operation on the image in Fig. 10a.
After running the thinning operation on Rsl, discontinuing contour may occur. For each end point P0 in Rt, the LASAO detector hence connects P0 and P1 if P1 is the nearest end point to P0 and the distance between P0 and P1 is less than Te. Let Re be the result after connecting the discontinuing object contours on Rt. Figure 11b is the obtained result by running the end point connecting on Fig. 11a. Similarly, the same LSA object will appear to multiple continuous MRI slices. The LASAO detector hence regards an object as a stent-LAS object if it also appears in previous or next chest MRI image. It means that if an object in Rl is overlapped with one object in previous image or next image, then the object is considered to the stent-LAS object, for example, the objects indicated by the red circles shown in Fig. 12. Let Rl be the result region obtained by this step.
In a stent-LAS object, the LAS object is encircled by the stent. The LASAO detector hence performs the erosion operation on Rl based on the structure element shown in Fig. 6b to remove the stent on Rl. Figure 13 demonstrates the segmented LAS object after removed the stent.
2.2 LAA object segmentation
This subsession is to extract the region containing the left aortic arch with stent; we call it the SLAA object. The LASAO detector first approximately locates the region containing the SLAA object; we name it the approximate SLAA region. The left aortic arch is located at the left side of the chest object. The LASAO detector hence sets the upper left corner and the lower right corner of the approximate SLAA region RA to (\( \overline{\boldsymbol{x}}-{\boldsymbol{l}}_{\boldsymbol{x}\boldsymbol{l}},\overline{\boldsymbol{y}}-{\boldsymbol{l}}_{\boldsymbol{y}\boldsymbol{u}} \)) and \( \left(\overline{\boldsymbol{x}}+\mathbf{1.25}\times {\boldsymbol{l}}_{\boldsymbol{x}\boldsymbol{r}},\overline{\boldsymbol{y}}+{\boldsymbol{l}}_{\boldsymbol{y}\boldsymbol{d}}\right) \). In Fig. 14a, the red dot is the gravity center (\( \overline{\boldsymbol{x}},\overline{\boldsymbol{y}} \)) of the chest object, and the red region is the approximate SLAA region. Figure 14b shows the segmented approximate SLAA region RA.
The LASAO detector converts the gray-level approximate SLAA region RA into a binary region RB based on the threshold obtained by Otsu thresholding method from the gray-levels of all the pixels in RA. It then removes the top and bottom bright objects in RB. After that, the opening operation with the structure element shown in Fig. 6b is used thrice to eliminate small bright spots in RB. Figure 15 illustrates the result after the image binarizing operation.
Furthermore, for each object in RB, the LASAO detector applies convex hull operation to draw the smallest convex polygon which encircles the whole object. The shape of the convex hull of the object can be described by formula (4), as well. If SL < TS, the object is removed from RB. The biggest one of the remaining objects in RB is considered to the SLAA object. Figure 16 shows the result obtained by the SLAA object segmentation. Finally, the erosion operation [8] with the structure element shown in Fig. 6b is used thrice to erode the stent in the SLAA object. Figure 17 displays the segmented LAA object.
2.3 Genetic based fittest parameters determining (GFPD) method
In this paper, a genetic based fittest parameters determining (GFPD) method is proposed to derive the most suitable values of the parameters Tg, r0, lxl, lxr, lyu, lyd, Ts, Ta, Te, and TS, which are used in the LASAO detector.
The GFPD method gives each chromosome 10 substrings sg, s0, sxl, sxr, syu, syd, ss, sa, se, and sS, respectively containing ng, n0, nxl, nxr, nyu, nyd, ns, na, ne, and nS binary bits and respectively corresponding to the decimal values vg, v0, vxl, vxr, vyu, vyd, vs, va, ve, and vS. Each chromosome hence composes of ng + n0 + nxl + nxr + nyu + nyd + ns + na + ne + nS bits and then Tg, r0, lxl, lxr, lyu, lyd, Ts, Ta, Te, and TS can be described as follows:
-
Tg = vg + 1,
-
\( \boldsymbol{r}\mathbf{0}=\raisebox{1ex}{${\mathrm{v}}_0$}\!\left/ \!\raisebox{-1ex}{${2}^{{\mathrm{n}}_0}$}\right.+0.005 \),
-
lxl = vxl,
-
lxr = vxr + 5,
-
lyu = vyu + 5,
-
lyd = vyd + 5,
-
\( \boldsymbol{Ts}=\raisebox{1ex}{${\mathrm{v}}_{\mathrm{s}}$}\!\left/ \!\raisebox{-1ex}{${2}^{\left({\mathrm{n}}_{\mathrm{s}}\right)}$}\right.+0.01 \),
-
Ta = va × 2 + 5,
-
Te = ve, and
-
\( \boldsymbol{TS}=\raisebox{1ex}{${\mathrm{v}}_{\mathrm{S}}$}\!\left/ \!\raisebox{-1ex}{${2}^{\left({\mathrm{n}}_{\mathrm{S}}\right)}$}\right.+0.01 \).
The GFPD method then executes the LASAO detector based on the parameters derived from the chromosome and the testing data are collected in advance. The obtained performance is used as the fitness of the chromosome.
In initial step, the GFPD method generates Nr chromosomes at random; we call the Nr chromosomes the reserved chromosomes. The GFPD method derives the best solution by repeatedly and alternately performing mutation, crossover, and selection operations [38] until the obtained best fitness of the chromosomes satisfies the requirement or the number of iterations equals to a given constant MAX_NO_ITER.
In crossover operation, the GFPD method randomly selects Nr chromosome pairs from the reserved chromosomes. The GFPD method gives a binary string SM for each chromosome pair (Ch1, Ch2), where SM comprises ng + n0 + nxl + nxr + nyu + nyd + ns + na + ne + nS bits. After that, the GFPD method randomly selects (ng + n0 + nxl + nxr + nyu + nyd + ns + na + ne + nS)/2 bits in SM and assigns all the selected bits to 1-bits, and others in SM to 0-bits. The GFPD method then creates a new chromosome Ch by the follow formula:
where ∩ represents the logical AND operator, ∪ the logical OR operator, and ~ the logical NOT operator.
For each reserved chromosome Ch, the mutation operation randomly indicates one bit b for each substrings in Ch, and ~ b is substituted for b to create a new chromosome. In this operation, Nr new chromosomes are hence generated.
The selection operation picks out 0.9 × Nr chromosomes with the best fitness from the reserved chromosomes and the chromosomes generated by the crossover as well as mutation operations. The selection operation randomly generates 0.1 × Ni chromosomes as well. Then the 0.9 × Ni selected chromosomes and 0.1 × Ni randomly created chromosomes are then used as the reserved chromosomes in next iteration.
3 Experiments
In SD, thirty-seven patients had no In-Stent restenosis and nine patients had In-Stent Restenosis after surgery. Additionally, thirty-seven patients in SD wore Assurance, and other nine patients were not put on Assurance; however, the nine patients with Assurance are not completely the same as the nine patients with the occlusion, as shown in Table 1.
Among the 9 patients without wearing Assurance, six patients had In-Stent Restenosis, and only three patients had no In-Stent Restenosis. Among the 37 patients wearing Assurance, only three patients had In-Stent Restenosis, and thirty-four patients had no In-Stent Restenosis. The statistical results illustrate that 66% of the patients with In-Stent Restenosis did not wear assurance, while 92% of the patients with assurance had no In-Stent Restenosis. It is quite obvious that wearing assurance can reduce the probability of causing In-Stent Restenosis.
Stent is an expandable device embedded into artery to open a blood vessel. An over-expanded stent may cause stent deformation or vessel fracture, and even the stent may lose its support [29]. There is no literature exploring the relationship between restenosis and the ratio of the cross-section diameter of stent to that of the left subclavian artery. This paper is hence to scrutinize the relationship between the cross-section diameters of the left subclavian artery and the selected stent on the basis of previous medical records regarding In-Stent Restenosis.
Assume that dS is the cross-section diameter of the stent employed in the stent surgery, ll is the distance between the two farthest pixels p1 and p2 on the contour of left subclavian artery before stenting, and lp is the distance of the two pixels q1 and q2 which are also located on the contour of the left subclavian artery. Additionally, line L΄, connecting q1 and q2, passes through the central point between p1 and p2 and is perpendicular to the line connecting p1 and p2.
The first experiment is to investigate the relationship between R = dS2/(ll × lp) and In-Stent Restenosis. Figure 18 shows the relationship between R = dS2/(ll × lp) and In-Stent Restenosis where the blue rectangles are the non-occlusion patients and the red triangles are the occlusion patients. Figure 18 demonstrates that the occurrence of In-Stent Restenosis can be decreased when given R > 0.82. Because the left subclavian artery of patients 22 and 39 are fractured, both patients inherently have a big chance of occurring In-Stent Restenosis after surgery.
All the images, each with 512 × 512 pixels, in SD were noted to occlusion or non-occlusion by some medical experts. The contours of LAS and LAA objects in each chest MRI images are also drawn by some medical experts, too; we consider the drawn objects the ground truth of the image. The data in SD are partitioned into two subsets Str and St: Str comprises 520 chest MRI images randomly selected from SD, and St consists of other 1182 chest MRI images in SD. In the following experiments, the data in Str are used as the training images, and those in St are used as the testing images.
In a data set, an ROI (region of interest) is a region identified for a particular purpose. In this paper, we call the segmented objects from the chest MRI images the detected ROIs. IoU (Intersection over Union) [47], Precision, Recall, F1-score, and Accuracy rate are often used evaluation metrics in measuring the performances of an object detector or object segmentation method on a particular dataset. They are defined as follows [12]:
where
-
TP (True positive): The number of the pixels which are in ground truth and also in the detected ROI by the LASAO detector,
-
FP (False positive): The number of the pixels which are not in ground truth but in the detected ROI by the LASAO detector,
-
TN (True negative): The number of the pixels which are neither in ground truth nor in the detected ROI by the LASAO detector,
-
FN (False negative): The number of the pixels which are in ground truth but not in the detected ROI by the LASAO detector,
-
and F1-score is the harmonic mean of precision and recall. In the following experiments, they will be used to describe the performances of the LASAO detector.
In the second experiment, the GFPD method is applied to evolve the fittest values of the parameters Tg, r0, lxl, lxr, lyu, lyd, Ts, Ta, and TS used in the LASAO detector based on the data in Str as well as ng = n0 = nxl = nxr = nyu = nyd = ns = na = ne = 6, MAX_NO_ITER = 20,000, and Nr = 10. In this experiment, the GFPD method obtains the fittest parameters Tg = 7, r0 = 0.025, lxl = 10, lxr = 45, lyu = 60, lyd = 30, Ts = 0.38, Ta = 15, Te = 5, TS = 0.51. Table 2 shows the performances of extracting the LAS and LAA objects from the chest MRI images in Str by the LASAO detector.
Furthermore, Table 3 demonstrates the performances of the LASAO detector in identifying whether the left subclavian artery is blocked or not based on the training data in Str and St. Here,
-
TP: The number of LSA objects, neither of which are occluded in ground truth and in the detected ROI by the LASAO detector,
-
FP: The number of LSA objects which are occluded in ground truth but are not occluded in the detected ROI by the LASAO detector,
-
TN: The number of LSA objects, which are occluded in ground truth and in the detected ROI by the LASAO detector,
-
FN: The number of LSA objects which are occluded in ground truth but are not in the detected ROI by the LASAO detector.
In this experiment, the Accuracy rate of detecting the blocked left subclavian arteries is used as the fitness of a chromosome in the GFPD method.
In the third experiment, the LASAO detector is employed to identify the obstructed left subclavian arteries for the chest MRI images in St based on the fittest parameters Tg = 7, r0 = 0.025, lxl = 10, lxr = 45, lyu = 60, lyd = 30, Ts = 0.38, Ta = 15, Te = 5, TS = 0.51 provided by the GFPD method. Tables 3 and 4 demonstrate the experimental results. The experimental results show that the LASAO detector offers almost the same results as the medical experts does. In the second experiment, the LASAO detector takes 5.2 seconds to train the fittest values of all the parameters used in the LASAO detector. The LASAO detector takes an average of 0.142 seconds to detect whether the subclavian artery is obstructed in a chest MRI image.
Deep learning has enabled lots of artificial intelligence applications lately in image classification [4, 7, 13, 15, 19, 22, 24, 27, 33, 40,42,43,43, 45] In this paper, the last experiment is to probe the performances of the deep learning method to detect the left subclavian artery and aortic arch from a chest MRI image. It is difficult for a convolutional neural network model to precisely classify the objects in an image with multiple scales as well as small sizes, and complicated image background [19, 43]. Many literatures [4, 24] have demonstrated that the fusion strategy of different convolutional layer feature maps can effectively solve the above problems.
An early fusion architecture [15, 27] integrates multiple data prior to analysis. It will face challenges when sampling rates differ between modes, or when some data sources are discrete and others are continuous. A late fusion architecture [29, 33, 42] employs some independent models to extract different features and fuses them in the decision stage. A late fusion architecture employs some independent models to extract different features and fuses them in the decision stage. Generally, late fusion architectures are much simpler than early fusion architectures, especially when there are large differences between data sampling rates, data dimensions, and measurement units. However, more computation is required for the late fusion architecture to train the larger number of classifiers. The intermediate fusion architecture [43] is the most flexible architecture that can fuse features at different stages of model training. An intermediate fusion architecture fuses different modalities into one hidden layer so that it can learn a joint representation of many modalities; it hence can greatly improve performances.
In [13, 41, 45], an extended feature pyramid network (EFPN) was proposed to the detect small objects with only a few pixels in an image. The EFPN employs pyramid network features to fuses the features of the objects with different sizes, and applies a feature texture transfer (FTT) module to super-resolve features and extracts credible regional details. Moreover, a cross resolution distillation mechanism is used to transfer the ability of perceiving details across the scales of the network.
In a chest MRI image, there exists many noise objects, such as aortic arch, left common carotid artery, brachiocephalic artery, thoracic spine, sternum, stent, and so on; additionally, the size of left subclavian artery is very small, with only a few pixels. Those mentioned above make the left subclavian artery and aortic arch extraction and recognition difficult. In this experiment, the EFPN [45] is hence employed to detect the left subclavian artery and aortic arch from a chest MRI image. In this experiment, the EFPN resized each image into an image of 512 × 512 pixels, and an NVIDIA RTX 2080 GPU with 1 image per mini-batch was used to train the network system. The parameters weight decay and momentum are respectively set to 0.0001 and 0.9; two constant parameters k0 and S0 are set to 4 and 224. The vertical context enlarge coefficient α and the horizontal context enlarge coefficient β are set to 0.15 and 0.9; γ is set to 0.9 which was used to adjust the expansion ratio between different datasets from factors. The total epochs of the model are set to 10. Table 4 demonstrates the experimental results.
Compared to the results in Table 2, one can obviously observe that the LASAO detector is much better than the EFPN in detecting the LAS and LAA objects from the chest MRI images. The main factors are that the shapes, sizes, locations, grayscale intensities of the LAS and LAA objects in different chest MRI images are much different. To make matters worse, many non-LAS and non-LAA objects frequently have very similar shapes, sizes, positions, and grayscale intensities with the LAS and LAA objects in chest MRI images, such as the images in Fig. 19 in which the yellow arrow indicates the LAA object while the red arrow indicates the LAS object.
4 Discussions and conclusions
For many patients requiring upper extremity access, the preferred route is from the left subclavian artery to the aorta. Unfortunately, it frequently encounters asymptomatic left subclavian artery occlusion and there is currently no good method for predicting restenosis after stenting of the left subclavian artery. In this paper, the LASAO detector is provided to extract the left subclavian arteries and the left aortic arches from chest MRI images, and then to identify the obstructed left subclavian arteries according to the gray-levels of the left subclavian artery and the left aortic arch. A chest MRI image contains tissues, blood vessels, bones, stents, and so on. Moreover, the left subclavian artery is very small. It makes the left subclavian artery and the left aortic arch segmentations difficult. The LASAO detector applies the color, spatial, temporal, and shape features to filter out the left subclavian artery and the left aortic arch. The experimental results have proven that the LASAO detector can give the impressive performances in segmenting the left subclavian arteries and the left aortic arches, and in identifying the obstructed left subclavian arteries. In this paper, the GFPD method is also proposed to evolve the most suitable values of the parameters used in the LASAO detector. However, the LASAO detector can only be used to detect whether the stented left subclavian artery occludes again.
In this paper, we also scrutinize the relationship between the cross-section diameter of the left subclavian artery and the size of the selected stent by using previous medical records. The experimental results show that a lower incidence of In-Stent Restenosis is provided for given C4 > 0.82, and the stent with assurance is helpful for preventing from In-Stent Restenosis.
Data availability
The datasets generated during and/or analysed during the current study are available in the [GoogleDrive] repository, [https://drive.google.com/file/d/1SY9TBLJDrFgsS48yGUQRnwgE6f0A84QH/view].
References
Aye CYL, Lewandowski AJ, Lamata P, Upton R, Davis E, Ohuma EO, Kenworthy Y, Boardman H, Wopperer S, Packham A, Adwani S, McCormick K, Papageorghiou AT, Leeson P (2017) Disproportionate cardiac hypertrophy during early postnatal development in infants born preterm. Pediatr Res 82:36–46
Baek JH, Kim BM, Yoo J, Nam HS, Kim YD, Kim DJ, Heo JH, Bang OY (2017) Predictive value of computed tomography angiography-determined occlusion type in stent retriever thrombectomy. Stroke 48(10):2746–2752
Bonati LH, Gregson J, Dobson J, McCabe DJH, Nederkoorn PJ, van der Worp HB, de Borst GJ, Richards T, Cleveland T, Müller MD, Wolff T, Engelter ST, Lyrer PA, Brown MM (2018) Restenosis and risk of stroke after stenting or endarterectomy for symptomatic carotid stenosis in the international carotid stenting study (ICSS): secondary analysis of a randomised trial. Lancet Neurol 17:587–596
Boulahia SY, Amamra A, Madi MR, Daikh S (2021) Early, intermediate and late fusion strategies for robust deep learning-based multimodal action recognition. Mach Vis Appl 32:121. https://doi.org/10.1007/s00138-021-01249-8
Charlick M, Das MJ (2021) Anatomy, head and neck, internal carotid arteries. Treasure Island (FL): StatPearls Publishing. https://pubmed.ncbi.nlm.nih.gov/32310521/
Chen X, Assadsangabi B, Hsiang Y, Takahata K (2018) Enabling angioplasty-ready “smart” stents to detect in-stent restenosis and occlusion. Adv Sci 5(5):1–10
Chen K, Franko K, Sang R (2021) Structured model pruning of convolutional networks on tensor processing units. arXiv:2107.04191v2 [cs.LG]. https://arxiv.org/abs/2107.04191
Chowdhury MM, Tarkin JM, Albaghdadi MS, Evans NR, Le EPV, Berrett TB, Sadat U, Joshi FR, Warburton EA, Buscombe JR, Hayes PD, Dweck MR, Newby DE, Rudd JHF, Coughlin PA (2019) Vascular positron emission tomography and restenosis in symptomatic peripheral arterial disease: a prospective clinical study. JACC Cardiovasc Imaging 13(4):1008–1017
Coffey S, Lewandowski AJ, Garratt S, Meijer R, Lynum S, Bedi R, Paterson J, Yaqub M, Noble JA, Neubauer S, Petersen SE, Allen N, Sudlow C, Collins R, Matthews PM, Leeson P (2017) Protocol and quality assurance for carotid imaging in 100,000 participants of UK biobank: development and assessment. Eur J Prev Cardiol 24(17):1799–1806
Crespo J, Schafer R (1994) The flat zone approach and color images. In: Serra J, Soille P (eds) Mathematical morphology and its applications to image processing. Kluwer academic publishers, Dordrecht, pp 85–92. https://doi.org/10.1007/978-94-011-1040-2_12
Cristianini N, Shawe-Taylor J (2000) An introduction to support vector machines and other kernel-based learning methods. Cambridge University Press. https://doi.org/10.1017/CBO9780511801389.013
Davis J, Goadrich M (2006) The relationship between precision-recall and ROC curves. Proceedings of the 23rd international conference on machine learning: 233-240. https://proceedings.neurips.cc/paper/2015/file/33e8075e9970de0cfea955afd4644bb2-Paper.pdf
Deng C, Wang M, Liu L, Liu Y, Jiang Y (2022) Extended feature pyramid network for small object detection. IEEE Trans Multimed 24:1968–1979
Dey D, Slomka PJ, Leeson P, Comaniciu D, Shrestha S, Sengupta PP, Marwick TH (2019) Artificial intelligence in cardiovascular imaging: JACC state-of-the-art review. J Am Coll Cardiol 73(11):317–1335
Gadzicki K, Khamsehashari R, Zetzsche C (2020) Early vs late fusion in multimodal convolutional neural networks. Proceedings of the 2020 IEEE 23rd International Conference on Information Fusion (FUSION):1–6. https://ieeexplore.ieee.org/stamp/stamp.jsp?tp=&arnumber=9190246
Goodfellow I, Bengio Y, Courville A, Bengio Y (2016) Deep learning 1. MIT Press, Cambridge
Guan X, Jian S, Hongda P, Zhiguo Z, Haibin G (2009) An image enhancement method based on gamma correction. The Second International Symposium on Computational Intelligence and Design (ISCID 2009) 1:60–63. https://ieeexplore.ieee.org/stamp/stamp.jsp?tp=&arnumber=5370400
Gunnoo T, Hasan N, Khan MS, Slark J, Bentley P, Sharma P (2016) Quantifying the risk of heart disease following acute ischaemic stroke: a meta-analysis of over 50,000 participants. BMJ Open 6(1):e009535. https://doi.org/10.1136/bmjopen-2015-009535
He Z, Cao Y, Du L, Xu B, Yang J, Cao Y, Tang S, Zhuang Y (2019) MRFN: multi-receptive-field network for fast and accurate single image super-resolution. IEEE Trans Multimed 22(4):1042–1054
Hellinger JC, Mendelson N, Pujata V, Kassam Z, Jahre C (2014) CT angiography: essential for evaluating atherosclerotic cardiovascular disease. Appl Radiol 43(7):10–19
Horiuchi Y, Tanimoto S, Latif AHMM, Urayama KY, Aoki J, Yahagi K, Okuno T, Sato Y, Tanaka T, Koseki K, Komiyama K, Nakajima H, Hara K, Tanabe K (2018) Identifying novel phenotypes of acute heart failure using cluster analysis of clinical variables. Int J Cardiol 262:57–63
Iwana BK, Uchida S (2019) Time series classification using local distance-based features in multi-modal fusion networks. Pattern Recogn 97:107024. https://doi.org/10.1016/j.patcog.2019.107024
Jarvis RA (1973) On the identification of the convex hull of a finite set of points in the plane. Inf Process Lett 2(1):18–21
Joze HRV, Shaban A, Iuzzolino ML, Koishida K (2020) MMTM: multimodal transfer module for CNN fusion. Proceedings of the IEEE/CVF conference on computer vision and pattern recognition: 13286-13296. http://arxiv.org/abs/1911.08670
Kelm BM, Mittal S, Zheng Y, Tsymbal A, Bernhardt D, Vega-Higuera F, Zhou SK, Meer P, Comaniciu D (2011) Detection, grading and classification of coronary stenoses in computed tomography angiography. Med Image Comput Computer-Assist Interv 14(pt 3):25–32. https://doi.org/10.1007/978-3-642-23626-6.pdf
Lancaster MC, Omar AMS, Narula S, Kulkarni H, Narula J, Sengupta PP (2019) Phenotypic clustering of left ventricular diastolic function parameters: patterns and prognostic relevance. JACC Cardiovasc Imaging 12:1149–1161
Liu H, Lu J, Yang H, Zhao X, Xu S, Peng H, Zhang Z, Niu W, Zhu X, Bao Y, Yan W (2020) Category-specific CNN for visual-aware CTR prediction at jd.com. Proceedings of the 26th ACM SIGKDD international conference on knowledge discovery and data mining: 2686-2696. https://doi.org/10.1145/3394486.3403319
Meijs M, Meijer FJA, Prokop M, Ginneken B, Manniesing R (2020) Image-level detection of arterial occlusions in 4D-CTA of acute stroke patients using deep learning. Med Image Anal 66(101810):1–7 https://reader.elsevier.com/reader/sd/pii/S1361841520301742?token=C34863652BACD108FA6716B77535CB07EE6421DC3211EC4E23BB4BD7CA03AFB2813CD472EF5B020608E80E430F4519A6&originRegion=us-east-1&originCreation=20220803153622
Migoń P, Duszyński F, Jancewicz K, Porębna W (2020) Late evolutionary stages of residual hills in tablelands. Geomorphology 367:1–16. https://doi.org/10.1016/j.geomorph.2020.107308
Nishie R, Toya N, Fukushima S, Ito E, Murakami Y, Misawa T, Ohki T (2019) Two cases of asymptomatic axillary artery occlusion difficult to diagnose preoperatively: pitfalls and its solution in endovascular therapy when approaching from the upper extremity. Surg Case Rep 5(121):1–4. https://doi.org/10.1186/s40792-019-0670-1
Niu G, Yan Z, Zhang B, Yang M (2020) Endovascular treatment of chronic total occlusion in the subclavian artery: a review of 23 cases. Front Neurol 11(264):1–7
Otsu N (1976) A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern 9(1):62–66
Pandeya YR, Lee J (2021) Deep learning-based late fusion of multimodal information for emotion classification of music video. Multimed Tools Appl 80(2):2887–2905
Sawicki M, Safranow K, Wiska L, Pasek I, Gajdziel A, Gruszewski M, Poncyljusz W (2021) Diagnostic value of artificial intelligence — based software in detection of large vessel occlusion in acute ischemic stroke. Appl Sci 11(21):1–8
Shlofmitz E, Iantorno M, Waksman R (2019) Restenosis of drug-eluting stents: a new classification system based on disease mechanism to guide treatment and state-of-the-art review. Circulation: Cardiovasc Interv 12(8). https://doi.org/10.1161/CIRCINTERVENTIONS.118.007023
Slomka PJ, Dey D, Sitek A, Motwani M, Berman DS, Germano G (2017) Cardiac imaging: working towards fully-automated machine analysis and interpretation. Expert Rev Med Devices 14(3):197–212
Suryawati E, Pardede HF, Zilvan V, Ramdan A, Krisnandi D, Heryana A, Yuwana RS, Kusumo RBS, Arisal A, Supianto AA (2021) Unsupervised feature learning-based encoder and adversarial networks. J Big Data 8(118):1–17
Syswerda G (1989) Uniform crossover in genetic algorithms. In: Schaffer JD (ed.) Proceedings of the third international conference on genetic algorithms. Morgan Kaufmann, San Mateo, 2–9:2–9
Tan LK, McLaughlin RA, Lim E, Aziz YFA, Liew YM (2018) Fully automated segmentation of the left ventricle in cine cardiac MRI using neural network regression. J Magn Reson Imaging 48(1):140–152
Tang S, Yu F (2021) Construction and verification of retinal vessel segmentation algorithm for color fundus image under BP neural network model. J Supercomput 77(4):3870–3884
Tran L (2021) EFPN: extended feature pyramid network for small object detection. Towards data science, Published by gbrewer. https://towardsdatascience.com/efpn-extended-feature-pyramid-network-for-small-object-detection-980af794a093
Trong VH, Gwang-hyun Y, Vu DT, Jin-young K (2020) Late fusion of multimodal deep neural networks for weeds classification. Comput Electron Agric 175:105506. https://doi.org/10.1016/j.compag.2020.105506
Wang S, Cong Y, Zhu H, Chen X, Qu L, Fan H, Zhang Q, Liu M (2020) Multi-scale context-guided deep network for automated lesion segmentation with endoscopy images of gastrointestinal tract. IEEE J Biomed Health Informa 25(2):514–525
Waszyk-Nowaczyk M, Guzenda W, Plewka B, Michalak M, Cerbin-Koczorowska M, Stryczyński Ł, Byliniak M, Ratka A (2020) Screening services in a community pharmacy in Poznan (Poland) to increase early detection of hypertension. J Clin Med 9(8):2572. https://doi.org/10.3390/jcm9082572
Wu Y, Tang S, Zhang S, Ogai H (2021) An enhanced feature pyramid object detection network for autonomous driving. Appl Sci 9(20):4363. https://doi.org/10.3390/app9204363
Zhang D, Park WJ, Lee SJ, Choi KA, Ko SJ (2012) Histogram partition baesd gamma correction for image contrast enhancement. The 16th IEEE international symposium on consumer electronics (ISCE 2012):1-4. https://ieeexplore.ieee.org/stamp/stamp.jsp?tp=&arnumber=6241687
Zheng Z, Wang P, Liu W, Li J, Ye R, Ren D (2020) Distance-IoU loss: faster and better learning for bounding box regression. The Thirty-Fourth AAAI Conf Artif Intell AAAI-20:12993–13000 https://ojs.aaai.org//index.php/AAAI/article/view/6999
Zreik M, Lessmann N, van Hamersvelt RW, Wolterink JM, Voskuil M, Viergever MA, Leiner T, Išgum I (2018) Deep learning analysis of the myocardium in coronary CT angiography for identification of patients with functionally significant coronary artery stenosis. Med Image Anal 44:72–85
Acknowledgments
The authors would like to acknowledge Ministry of Science and Technology, Taiwan, R.O.C. for the financial support provided.
Funding
The research leading to these results received funding from Ministry of Science and Technology, Taiwan, R.O.C. under Grant Agreement No. 110–2221-E-005-077.
Author information
Authors and Affiliations
Contributions
YKC and YCL conceived the study. WJW and WTH designed the approach and performed the computational analysis with YKC and CHL. YKC, YCL, and CHL supervised the work and tested the program. YKC, YCL, CHL, CHL, WJW, and WTH wrote the manuscript. YCL prepared the samples and collected the data. YKC, YCL, and CHL contributed analyzing experimental studies. All authors read and approved the final manuscript. YKC, YCL, and CHL contributed equally; YKC and YCL are the first authors, YKC and CHL are the correspondents as well as listed in alphabetical order.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chan, YK., Lin, YC., Wang, WJ. et al. Identifying the occlusion of left subclavian artery with stent based on chest MRI images. Multimed Tools Appl 82, 10617–10639 (2023). https://doi.org/10.1007/s11042-022-13735-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11042-022-13735-w