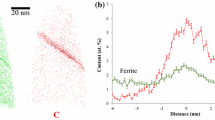
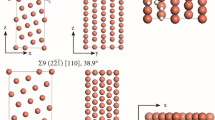
A model of the arrangement of atoms of interstitial impurity in iron austenite is developed, according to which an introduction of an interstitial impurity atom causes transformation of a regular close packing (CP or FCC) composed of octahedrons and tetrahedrons into a cementite-type local prismatic packing by diagonal flipping. Carbon dissolves in the crystalline structure of iron austenite as a centered five-atom tetrahedral cluster, which represents a fragment of the carbon packing in the crystalline structure of diamond. The diffusion of the impurity atom is accompanied by a transformation into a prism in the neighboring lattice period, while the prismatic packing in the initial location of the impurity atom is reconstructed back into a conventional interstitial void. To check the adequacy of the proposed model, theoretical calculations of the activation enthalpy of carbon and nitrogen diffusion in austenite were performed, according to which the activation enthalpy values are 149 ± 20 and 155 ± 20 kJ/mol, respectively, which is fairly consistent with the experimental data reported in the literature (153 and 169 kJ/mol).






Similar content being viewed by others
References
B. Cordero, V. Gomez, A. E. Platero-Prats, et al., “Covalent radii revisited,” Dalton Trans., No. 21, 2832 – 2838 (2008); https://doi.org/10.1039/B801115J.
B. D. Butler and J. B. Cohen, “The location of interstitial carbon in austenite,” J. Phys. I France, 2, 1059 – 1065 (1992); https://doi.org/10.1051/jp1:1992195.
P. Pyykkö and M. Atsumi, “Molecular double-bond covalent radii for elements Li–E112,” Chem. Eur. J., 15, 12770 – 12779 (2009); https://doi.org/10.1002/chem.200901472.
K. H. Jack, “The iron-nitrogen system: the preparation and the crystal structures of nitrogen-austenite (γ) and nitrogen-martensite (α‘),” Proc. Royal Soc. London, Ser. A. Math. Phys. Sci., 208(1093), 200 – 215 (1951); https://doi.org/10.1098/rspa.1951.0154.
J. W. Martin, Precipitation Hardening, Butterworth-Heinemann, Oxford (1998).
H. K. D. H. Bhadeshia, S. A. David, J. M. Vitek, and R.W. Reed, “Stress induced transformation to bainite in Fe – Cr – Mo – C pressure vessel steel,” Mater. Sci. Technol., 7, 686 – 698 (1991); https://doi.org/10.1179/mst.1991.7.8.686.
R. Kojima and M. Susa, “Melting of thin Fe – C films having (100), (110) and (111) surfaces in terms of molecular dynamics simulation,” Sci. Technol. Adv. Mater., 5, 677 – 682 (2004); https://doi.org/10.1016/j.stam.2004.03.011.
R. R. Reeber and K. Wang, “Thermal expansion, molar volume and specific heat of diamond from 0 to 3000 K,” J. Electron. Mater., 25, 63 – 67 (1996); https://doi.org/10.1007/BF02666175.
V. S. Kraposhin, A. L. Talis, and M. I. Samoylovitch, “Axial (helical) substructures determined by the root lattice E8 as generating clusters of the condensed phases,” J. Non-Cryst. Solids, 353, 3279 – 3284 (2007); https://doi.org/10.1016/j.jnoncrysol.2007.05.065.
V. S. Kraposhin, N. D. Simich-Lafitskiy, A. L. Talis, et al., “Formation of the cementite crystal in austenite by transformation of triangulated polyhedral,” Acta Crystallogr. B. Struct. Sci. Cryst. Eng. Mater., B75, 325 – 332 (2019); https://doi.org/10.1107/S205252061900324X.
A. L. Talis, V. S. Kraposhin, S. Y. Kondrat’ev, et al., “Non-crystallographic symmetry of liquid metal, flat crystallographic faults and polymorph transformation of the M7C3 carbide,” Acta Cryst., Sec. A: Found. Adv., A73, Pt. 3, 209 – 217 (2017); https://doi.org/10.1107/S2053273317000936.
D. Pettifor, in: R.W. Cahn and P. Haasen (eds.), Physical Metallurgy, Elsevier, Amsterdam (1996), Vol. 1, pp. 47 – 133.
P. D. Desai, “Thermodynamic properties of iron and silicon,” J. Phys. Chem. Ref. Data, 15(3), 967 – 983 (1986); https://doi.org/10.1063/1.555761.
H. M. Ledbetter and R. P. Reed, “Elastic properties of metals and alloys. I. Iron, nickel, and iron-nickel alloys,” J. Phys. Chem. Ref. Data, 2(3), 531 – 618 (1973); https://doi.org/10.1063/1.3253127.
J. J. Wits, T. A. Kop, Y. van Leeuwen, et al., “A study on the austenite-to-ferrite phase transformation in binary substitutional iron alloys,” Mater. Sci. Eng. A., 283(1 – 2), 234 – 241 (2000); https://doi.org/10.1016/S0921-5093(00)00735-8.
M. Hillert and L. Höglund, “Mobility of α/γ-phase interfaces in Fe alloys,” Scr. Mater., 54, 1259 – 1263 (2006); https://doi.org/10.1016/j.scriptamat.2005.12.023.
C. Zener, in: W. Shockley, J. H. Holomon, R. Maurer, and F. Seitz (eds.), Imperfections in Nearly Perfect Crystals, John Wiley & Sons, NewYork (1952), pp. 289 – 314.
A. D. Le Claire, in: H. Mehrer (ed.), Diffusion in Solid Metals and Alloys, Springer-Verlag, Landolt–Bornstein new series (1990), Vol. III(26), pp. 471 – 503.
V. G. Gavriljuk, “Carbon and nitrogen in iron-based austenite and martensite: An attempt at comparative analysis,” J. Phys., 112, 51 – 59 (2003); https://doi.org/10.1051/jp4:2003839.
I. Fall and J. M. Génin, “Mössbauer analysis of Fe – N austenites,” Hyperfine Interact., 69, 513 – 516 (1992); https://doi.org/10.1007/BF02401877.
P. Bauer, O. N. C. Uwakweh, and J. M. R. Genin, “Cems study of the carbon distribution in austenite,” Hyperfine Interact., 41, 555 – 558 (1998); https://doi.org/10.1007/BF02400451.
N. De Cristofaro and R. Kaplow, “Interstitial atom configurations in stable and metastable Fe – N and Fe – C solid solutions,” Metall. Mater. Trans. A, 8, 35 – 44 (1977); https://doi.org/10.1007/BF02677261.
B. M. Mogutnov, I. A. Tomilin, and L. A. Shvartsman, Thermodynamics of Iron-Carbon Alloys [in Russian], Metallurgiya, Moscow (1972).
H. Schenck and H. Kaiser, “Untersuchungenüber die Aktivität des Kohlenstoffs in kristallisiertenbinären und ternären Eisen-Kohlenstoff-Legierungen,” Arch. Eisenhüttenw., 31, 227 – 235 (1960); https://doi.org/10.1002/srin.196002854.
I. A. Tomilin, “On the relationship between the thermodynamic properties of austenite and its structure,” Dokl. Akad. Nauk SSSR, Ser. Khim., No. 162, 384 – 387 (1965).
K. Schubert, Kristallstrukturen Zweikomponentiger Phasen, Springer Verlag, Berlin (1964).
M. L. Fornasini and M. Pani, “Ba5Ga6: a phase with octahedral clusters of gallium,” J. Alloys Compd., 205, 179 – 181 (1994); https://doi.org/10.1016/0925-8388(94)90786-2.
This study was sponsored by the Russian Foundation for Basic Research (project No. 19-02-00085).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 5, pp. 10 – 17, May, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Semenov, M.Y., Kraposhin, V.S., Arestov, V. et al. Arrangement of Atoms of Interstitial Impurity in the Crystal Lattice of Iron Austenite and Mechanism of their Diffusion Jump. Met Sci Heat Treat 65, 272–278 (2023). https://doi.org/10.1007/s11041-023-00925-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-023-00925-y