Abstract
Calcium (Ca2+) is a universal signaling molecule that is tightly regulated, and a fleeting elevation in cytosolic concentration triggers a signal cascade within the cell, which is crucial for several processes such as growth, tolerance to stress conditions, and virulence in fungi. The link between calcium and calcium-dependent gene regulation in cells relies on the transcription factor Calcineurin-Responsive Zinc finger 1 (CRZ1). The direct regulation of approximately 300 genes in different stress pathways makes it a hot topic in host-pathogen interactions. Notably, CRZ1 can modulate several pathways and orchestrate cellular responses to different types of environmental insults such as osmotic stress, oxidative stress, and membrane disruptors. It is our belief that CRZ1 provides the means for tightly modulating and synchronizing several pathways allowing pathogenic fungi to install into the apoplast and eventually penetrate plant cells (i.e., ROS, antimicrobials, and quick pH variation). This review discusses the structure, function, regulation of CRZ1 in fungal physiology and its role in plant pathogen virulence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Host-pathogen interaction is a key step in pathogenic development. Since the progression of the infection is based on different, discrete, steps, fine-tuning of various pathways is required especially in pathogen physiology as a response to host defense and the presence of a “master regulator” that precisely regulate the transcriptomic mechanism represent a focal point of the pathogen life. Because information is transmitted via signaling pathways that are not simple ON – OFF responses, modulation is achieved through a frequency modulator [1] based on oscillations in cytosolic calcium (Ca2+) concentrations, which act as ubiquitous signals and control a wide array of cellular functions [2]. Moreover, a transient increase in intracellular calcium levels is crucial for cell adaptation to external stress via the activation of the calmodulin (CaM)/ calcineurin (CaN) signaling pathway [3], which triggers (via dephosphorylation) the entry of the transcription factor Calcineurin-Responsive Zinc finger 1 (CRZ1) into the nucleus [4]. Here, the presence of a specific consensus sequence in the target gene promoter is defined as CDRE (calcineurin-dependent responsive element) through which the organism can mediate the transcriptomic response to adapt to external stimuli [5].
This review focuses on the structure of CRZ1, its role in the calcium cell survival (CCS) pathway, which is involved in a variety of life processes, and how it fits into the host-pathogen crosstalk. However, like the sorites paradox of when a heap of sand became only a grain, the singular events have already been revised in depth, but the role of CRZ1 in the inter-communication process is still not completely clear. Notably, pathogenic fungi must face a hostile and changing environment; from penetration to cell exploitation, the pathogen encounters several environmental insults, such as increased ROS concentration, alkalinization of the apoplast, and toxic phenolics inter alia. Adapting to this fast-changing environment requires tight control of gene expression and specifically of those genes involved in adaptation, such as antioxidation and detoxification, but concomitantly triggers the expression of genes related to virulence, which is a matter of simultaneous defense and attack. We believe that CRZ1 could play a leading or important role in orchestrating this strategy.
Calcineurin responsive zinc finger CRZ1
The transcription factor CRZ1 belongs to the super-category of the zinc finger transcription factor (ZF-TF) and carries out its regulatory action through zinc finger motifs (ZF).Those domains are composed of one α-helix and two antiparallel β-strands, which also allows its categorization into different categories: the Cys2His2 family, that embraces hundreds of these proteins in eukaryotes, from yeast to humans [6], and the zinc cluster protein family (C6) that is only present in fungi with the unique CysX2CysX6CysX5-12CysX2CysX6-8Cys motif. In particular, the cysteine residues bind two zinc atoms to coordinate the correct folding of the DNA-binding domain [7], contributing to the correct protein function [8].
CRZ1 belongs to the C6 family: a zinc finger with a DNA-binding domain (DBD) and six cysteine residues bound to two zinc atoms, categorized as a zinc cluster (ZFC), zinc binuclear cluster, or Zn(II)2Cys6 (Zn2C6) proteins. Those proteins have only one zinc finger domain with two zinc atoms, and shows the ability to bind DNA as monomers, homodimers, or heterodimers. ZFC proteins have been identified exclusively in fungi and are distributed at a ratio of 10:20:40 in chytrids-zygomycetes, basidiomycetes, and ascomycetes, respectively [9], although other ZFC proteins are also present [7]. The C6 family protein includes the transcription factor Gal4 of Saccharomyces cerevisiae [10], in which these six cysteine residues bind to two Zn(II) ions in a bimetal thiolate cluster [7]. Different functional domains have been identified in this protein, including a DNA-binding domain (DBD; residues 1–65), a dimerization domain (residues 65–94), three acidic activation domains, and a C-terminal region that interacts with the inhibitor Gal80p. The DBD consists of three regions: zinc finger, linker, and dimerization regions [7] (Fig. 1).
The site responsible for the Zn bond has two further “sub-structures” each formed by 3 Cys surrounded by basic amino acids and separated by a loop, which together form two short α-helices within which the two zinc atoms are positioned consequently bonded to 6 total Cys residues. This portion is normally found in the N terminal region [11]. The linker region, the most variable part of the protein, allows its extension on ssDNA, binding the phosphodiester backbone and generating an extremely rigid “scaffold” preventing the binding to off-target sequences [12]. In general, the dimerization region contains seven repeats, forming a conserved coiled-coil structure responsible for protein-protein interactions. The lack of this region, as in Ume6p of S. cerevisiae, indicates monomeric behavior [13]. Zn (II) 2Cys6 - DNA-binding domains typically interact with DNA-binding sites formed by conserved terminal trinucleotides. These trinucleotides feature a symmetrical arrangement and are separated by an internal sequence of variable lengths, ranging from 2 to 17 nucleotides. It is worth noting that these DNA-binding domains primarily interact with specific sections of the DNA [14] called, in CRZ1’s case, calcineurin dependent responsive elements or CDRE [15].
CRZ1’s passepartout: the calcineurin dependent responsive element (CDRE)
The consensus motif
The zinc finger domain of CRZ1 engages with the 24 bp CDREs sequence to initiate the expression of target genes [4]. In S. cerevisiae, the primary consensus site for CRZ1 binding is 5′-GNGGCKCA-3′ [16], whereas the proposed common DNA sequence bound by CRZ1 in Trichoderma reesei is 5′-GDGGCKBNB-3′ [17]. A recent analysis of promoters in Candida albicans revealed a distinct consensus motif [5′-GGAGGC(G/A)C(T/A)G-3′] that diverged from the putative CaCRZ1-binding motif [5′-G(C/T)GGT-3′] identified in a previous research [18]. In-depth studies have revealed that zinc cluster proteins recognize closely related elements, encompassing trinucleotide sequences presented either singly or in repeated patterns, whether symmetrical or asymmetrical. While CGG triplets are prevalent, variations within these binding elements have been documented, ensuring the capacity of each protein to perform unique regulatory functions [7]. As noted earlier, several factors exert influence over DNA targeting and binding of zinc cluster proteins. Within the protein structure, numerous components of the DNA-binding domain contribute significantly to interaction with the target DNA. Additionally, the nucleotides encompassing CGG triplets also exert a measure of influence on DNA-binding affinities [19]. However, two crucial factors that determine DNA-binding specificity are the orientation of CGG triplets and the distance between them.
The target genes in fungi
CRZ1 governs a range of target genes that are responsible for maintaining ion equilibrium, sustaining cell wall integrity, driving lipid synthesis, managing protein degradation, and overseeing glucose metabolism. Its influence extends to species-specific genes, serving as a controller of both gene expression induction and inhibition. Additionally, CRZ1 is indispensable for orchestrating the response of the two P-type ATPases, PMC and PMR, to calcium ions to facilitate Ca2+ translocation from the cytoplasm to the vacuoles and Golgi apparatus. This finely tuned transport safeguards the intracellular calcium balance [20].
In yeast pmc and pmr gene expression displays an increased in response to Ca2+ stimuli. However, CRZ1 mutants exhibit reduced activation of these genes [16, 21]. This attenuation leads to disrupted calcium equilibrium, potentially explaining the calcium sensitivity observed in CRZ1 mutants. CRZ1 dependence extends to other genes such as ena1, ena2, and ena3, coding for plasma membrane Na+/Li+-ATPases that ensure yeast viability at elevated concentrations of these ions. Their induction depends on CaN and CRZ1 interactions [22]. The CaN-CRZ1 pathway also regulates ion homeostasis-related genes such as mep1, enb1, pho84, pho89, and kha1 [16]. In encounters with external stressors, cell wall integrity relies on β-1,3 glucan synthase (FKS) and chitin synthase (CHS). Disruption of CRZ1 prompts irregular expression of fks and chs [23].
In plant pathogens, as Aspergillus flavus, the CaN-CRZ1 axis extends its influence on other cell wall maintenance genes such as crh1, rho1, scw10, and kre6 [14]. Notably, CRZ1’s role in Penicillium oxalicum encompasses cellulase synthesis regulation through genes such as cbh1, eg1, and eg2 [24].
Furthermore in A. flavus, lipid and sterol metabolism-associated genes (sur1, csg2, ysr3, erg26, hes1, and plb3), along with vesicular transport-related genes (gyp7, ypt53, yip3, pep12, rvs161, she4, cvt17, cvt19, and vps36), are also CRZ1-regulated. Collectively, these genes enable proficient membrane function and efficient substance delivery to the cell surface [14]. These evidences in plant pathogenic fungi show the role of calcium homeostasis as pivotal for orchestrating effective responses to diverse stimuli.
IN-N-OUT: mechanism of CRZ1 activation
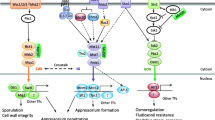
Calcium signaling plays a pivotal regulatory role throughout fungal growth and development. Defects in this pathway trigger anomalies in different facets of fungal cells, including reproductive development, polar growth, cell differentiation and division, stress responses, and programmed cell death [25]. Consequently, preserving the intracellular calcium balance is a crucial factor in determining cell survival. Under normal physiological conditions, the fungal cell cytoplasm harbors a modest concentration of Ca2+ ranging from 50 to 100 nM [25]. Eukaryotes manage cellular calcium stability through an intricate regulatory system that involves diverse Ca2+ channel proteins, pumps, transporters, and relevant enzymes [26]. Positioned predominantly on the plasma membrane or within various subcellular organelles, these constituents collaboratively manage Ca2+ intake from extracellular and intracellular reservoirs, thereby orchestrating the equilibrium between cytoplasmic and organelle-specific Ca2+ levels [27]. The calcium/calcineurin pathway, a well-conserved element spanning from mammals to yeasts [28], involves calcineurin, a phosphatase that triggers stress responses and preserves drug resistance. This function is enacted through protein-protein interactions or genetic interplays involving CRZ1, which governs the activation of over 100 genes [29]. Although calcium bursts are recurring observations in various cell types, such as yeast [30], the relationship between cytosolic calcium concentration and CRZ1 localization within individual cells is yet to be scrutinized. Mechanistic models of CRZ1 regulation via calcium signaling do not anticipate the oscillation of cytoplasmic calcium concentration ([Ca2+]cyt) induced by variations in the external calcium concentration ([Ca2+]ext) [31]. Additionally, although the average nuclear localization of CRZ1 increases with elevated [Ca2+]ext [32], [Ca2+]cyt is rigorously regulated and maintains similarity across a broad range of [Ca2+]ext [33]. This discrepancy implies that the frequency of CRZ1 pulsatility, which heightens with [Ca2+]ext rising [32], is unlikely to mirror the variance in the average [Ca2+]cyt. Hsu et al. [1] posited that the connection between [Ca2+]cyt and CRZ1 pulsations resembles that of a noisy analog-to-digital converter: heightened calcium bursts correspond to increased CRZ1 pulses. Prior investigations of CRZ1 pulsatility suggest that CRZ1 pulses are actively generated, rather than passively tracking fluctuations in [Ca2+]cyt [32]. Instead, individual CRZ1 molecules have been proposed to gauge [Ca2+]cyt with time delay. This interpretation potentially elucidates the observed correlation between a high affinity calcineurin docking site on CRZ1 and an increased pulsing frequency [32], where greater affinity facilitates longer oscillations following a calcium burst. A proteome-wide screen was conducted using movies to systematically identify localization-based pulsing behaviors in Saccharomyces cerevisiae [34], analyzing all genes in a previously developed library of 4,159 strains containing fluorescent protein fusions [22]. Interestingly, CRZ1 is the only found nonredundant or paralogous pulsed transcription factor. The prevalence of pulsing in various biological systems across different species implies that pulsing could be a widespread solution to many biological challenges. Indeed, it has been shown to proportionally regulate entire regulons of target genes [32] and providing a temporal mode of regulation that facilitates this and other functions [35]. Calcineurin interacts with the transcription factor through the calcineurin docking domain PIISQ [36] and a serine-rich region housing multiple serine residues. This region serves as a target for CRZ1 dephosphorylation, influencing its localization and phosphorylation levels [37]. In the absence of external stress or other stimuli, CRZ1 resides in the cytoplasm. However, elevated Ca2+ concentrations trigger CaN activation, leading to CRZ1 dephosphorylation and its subsequent migration to the nucleus for the regulation of target genes (Fig. 2a).
CRZ1 shuffling dynamics arise from the presence of both a nuclear localization signal (NLS) and nuclear export signal (NES). In quiescent cells, CRZ1 predominantly occupies the cytosol, because the prevailing rate of nuclear export exceeds that of nuclear import. Upon the activation of calcineurin-dependent signaling, CRZ1 swiftly relocates to the nucleus, thereby initiating gene expression. The dephosphorylation of CRZ1p induces a conformational alteration that unveils its NLS, allowing it to interact with importin NMD5p [38]. This dephosphorylation also inhibits the interaction between CRZ1’s NES and the export receptor MSN5p, thereby curbing nuclear export. Calcineurin-mediated dephosphorylation of CRZ1 serves a dual purpose: to increase the nuclear import rate and diminish the nuclear export rate. These combined effects expedite CRZ1’s nuclear accumulation upon calcineurin activation, with continuous calcineurin-mediated dephosphorylation “vital” for maintaining CRZ1’s nuclear localization. The termination of signaling prompts CRZ1’s rapid cytosolic return, driven by its re-phosphorylation [37]. However, in species such as C. glabrata and C. neoformans, CRZ1 activation occurs through a calcineurin-independent route [4]. In one instance, a microarray analysis identified 33 genes under CRZ1 regulation via a calcineurin-independent mechanism. Of these, 16 were upregulated and 17 were downregulated upon exposure to the calcineurin-inhibiting drug FK506 [39]. In C. dubliniensis, CRZ1-directed control of thigmotropism is calcineurin-independent [40]. S. cerevisiae shows a distinct angle, where the kinase HRR25, with roles encompassing the DNA damage response, mitosis, and vacuole transport, engages with CRZ1, phosphorylating it to elicit alterations in its localization [41]. The ability to modulate diverse stress response pathways positions CRZ1 as a focal point in plant pathology research, given its multifaceted regulatory involvement in governing virulence within host-pathogen interactions.
CRZ1-mediated response to host stressors
The stressors inherent in host-pathogen interactions encompass a spectrum of variations, including oxidative stress, pH fluctuations, and interference agents targeting the cell wall. In response, fungi have evolved diverse strategies to swiftly perceive these signals and mitigate the resulting damage. The transcription factor CRZ1 has emerged as a pivotal player, activated by the surge in Ca2+ levels induced by stress, orchestrating the regulation of pertinent gene expression. Indeed, CRZ1 mutants exhibit heightened sensitivity to ion stress owing to the translocation of dephosphorylated CRZ1 into the nucleus, triggering the expression of multiple genes involved in calcium ion stress responses, such as pmc and pmr [16]. However, susceptibility to cation ion stressors, like Na+, Li+, Mg2+, and Mn2+, varies across fungal species with CRZ1 deletions. For instance, in Aspergillus fumigatus, the absence of CRZ1 leads to high sensitivity to Mn2+ but diminished sensitivity to Na+ and Li+. In contrast, in Magnaporthe grisea the knockout of ∆Crz1 was insensitive to Na+, Li+, and Mn2+ [55]. Conversely, in Botrytis cinerea the absence of Crz1 gene generates mutant with a pronounced sensitivity to these four ion stresses; however, the addition of Mg2+ restored growth impairments and cell wall integrity [42]. These insights underscore the commonality between CRZ1-regulated ion stress responses and ion homeostasis in fungi, albeit with species-specific nuances. Evidently, CRZ1’s sensitivity to oxidative stress transcends species barriers, as has been confirmed in various organisms including B. cinerea [42], Metarhizium acridum [56], and Penicillium digitatum [56] (Fig. 2). Furthermore, CRZ1’s role in yeast tolerance to elevated pH levels is well established. Alkaline conditions elicit the expression of alkaline pH-responsive genes, such as ena1, pho84, pho89, and pho12 [58]. Under extreme pH levels (pH 3 or 9), ΔBcCrz1 exhibits diminished colony growth rate, akin to ion stress scenarios, and exogenous Mg2+ supplementation restores growth at pH 9, although the growth defect remains unresolved at pH 3 [42]. Another facet of the ΔCrz1 mutant is its heightened sensitivity to cell wall inhibitors. Notably, the growth of CRZ1 mutants in P. digitatum, Magnaporthe oryzae, and B. cinerea is markedly impaired in media containing SDS, CR, or CFW [42, 54, 57]. Interestingly, in Valsa pyri, ∆VpCrz1 exhibited significantly increased mycelial growth on CM agar medium containing SDS, CR, or CFW compared with the wild-type strain, diverging from previous reports [47]. Correspondingly, the CRZ1 mutant showed SDS resistance in the human pathogenic fungus Candida lusitaniae, suggesting CRZ1’s negative regulation of cell membrane integrity. Intriguingly, its response to SDS operates through an unknown mechanism, independent of CaN [59].
The bad, the ugly and the CRaZy: involvement in pathogenesis of plant fungi
As previously elucidated, the CSS signaling cascade maintains sway over fungal growth, development, and pathogenicity. The absence of CRZ1 causes aberrations in the vegetative growth of most plant pathogenic fungi (Fig. 2b). A prime example is the ∆BcCrz1 mutant of Botrytis cinerea, which exhibits compromised mycelial growth and unusual branching in CM medium [42]. In addition, Fusarium graminearum and Aspergillus nidulans hinder vegetative growth following CRZ1 loss [41, 42]. Crucial to fungal life, conidia formation and development are intimately linked to CRZ1. For instance, ∆BcCrz1 fails to generate sporophores or conidia [42, 43], whereas ∆FgCrz1 presents impaired perithecium formation, thereby affecting sexual development [44]. A noteworthy instance is observed in A. nidulans, where a pressure-sensing mechanism on the cell wall triggers calcium channel opening, activating the CNA/CRZ1 complex and propelling mycelial polar growth. Beyond this, CRZ1’s role extends widely, encompassing mycelial growth, morphological transformation, spore and appressorium formation, and serving as a precursor for pathogenicity [4]. In Magnaporthe oryzae, CRZ1 deleted strains exhibit reduced pathogenicity, which is mainly attributed to decreased appressorium swelling pressure, resulting in osmotic damage owing to disrupted lipid metabolism [45]. Verticillium dahliae, implicated in Verticillium wilt disease in smoke trees [46], generates melanized microsclerotia and in the ΔVdCrz1-2 mutant, microsclerotia formation was markedly compromised, with accumulated melanin and increased fragility [47]. Furthermore, expression of the hydrophobin gene vdh1 is pivotal for early microsclerotia development [48] and, with other melanin biosynthesis genes, is significantly downregulated in ΔVdCrz1-2. The addition of Mg2+ to low-Mg2+ basal medium restores the abnormal microsclerotia phenotype, underscoring the pivotal role of the calcineurin-CRZ1 signaling machinery in host plant infection [47]. P. digitatum, a citrus fruit pathogen, exhibits diminished virulence and a ~ 35% reduction in lesion size due to the ∆PdCrz1 mutation [49]. V. pyri, which inflicts canker disease on apples and pears [50], witnesses reduced diameter of wounded pear fruits and 1-year-old branches in the ΔVpCrz1 mutant. The attenuated virulence is attributed to the CRZ1/MAPK signaling cascade [51]. In (B) cinerea, the ΔBcCrz1 mutant experiences compromised cell wall integrity, leading to defective mycelium-derived infection and reduced lesion diameter in infected bean plant leaves. However, as reported for the V. dahliae CRZ1 mutant, Mg2+ supplementation enhanced cell wall stability in ΔBcCrz1 hyphae and mitigated penetration defects [42]. F. graminearum, the causative agent of Fusarium head blight (FHB) in wheat and cereals, generates mycotoxins such as deoxynivalenol (DON) and zearalenone (ZEA) in infected grains [51, 52]. The ΔFgCrz1A mutant showcases attenuated virulence, struggles to propagate in a neighboring spikelet, and displays reduced expression of the DON biosynthesis gene tri, resulting in decreased DON production [44]. In M. oryzae, lipid droplet transportation from conidia to nascent appressorium is disrupted in ΔMoCrz1, leading to a decreased appressorial penetration rate and early lipid droplet degradation upon infection [53, 54]. While CRZ1 indeed assumes a conserved role in fungal virulence or pathogenicity, distinct mechanisms govern these effects, as revealed through studies on fungal pathogen infection mechanisms.
a, regulation of CRZ1 in the presence of environmental stressors (left panel). The perception of signals leads to the accumulation of Ca2+ in the cytoplasm through the opening of Ca2+ channels (e.g., in the endoplasmic reticulum or vacuole). This process activates calmodulin (CaM), which phosphorylates calcineurin, which in turn, dephosphorylates CRZ1 that shuffles into the nucleus to activate the transcription of target genes (Created with BioRender.com.), b, list of cellular functions and pathogenesis implication of CRZ1 in plant pathogenic fungi
Relation between CRZ1 and mycotoxin biosynthesis
Mycotoxins are harmful humans/animals’ secondary metabolites produced by filamentous fungi. Defining their ecological role is challenging. Only in a few cases, among which aflatoxins and deoxynivalenol, their role in fungal lifestyle has been suggested: aflatoxins should provide stationary-phase A. flavus colonies with an extended lifespan owing to their high antioxidant abilities [60], while deoxynivalenol appears to be crucial for rachis penetration during F. graminearum colonization of ovary tissues into wheat spikelets [61]. Interestingly, the synthesis of both mycotoxins is apparently controlled by CRZ1. In F. graminearum, the lack of CRZ1 (in the ΔFg01341 mutant) causes dramatic defects in the ability to colonize flowering wheat heads and corn silks, which is consistent with the reduction in deoxynivalenol production demonstrated in this strain [44] (Fig. 2b). In A. flavus, deletion of the CRZ1-orthologue crzA causes a severe impairment in the ability to colonize maize kernels and aflatoxins production [62]. These few examples suggest us that CRZ1 by exerting a role also in mycotoxin synthesis could effectively represent a “hub” for orchestrating general responses to stresses: specifically, the host poses various threats to fungal survival, forcing responses that requires the correlation among diverse pathways int pathogenic fungi some of which (e.g., mycotoxins) are not forcedly or closely related to virulence.
Conclusions
As a vital transcription factor in the calcium signaling pathway, CRZ1 is extensively preserved in fungi and performs a crucial function in growth, development, stress tolerance, and pathogenicity. The link between stress response and transcriptional regulation makes CRZ1 a key element in the survival mechanism of the pathogen, particularly in adverse conditions, such as the interface with the host. CRZ1 can regulate different responses, particularly which gene cluster; however, some pieces are missing. The presence of a small binding motif, where only three base pairs seem to be fundamental, makes analysis very difficult, and an in silico approach can be misleading and limited in identifying subtle changes in complex binding motifs under different conditions. To overcome this limitation, it is crucial to use tools that enable sensitive identification with a single base-pair resolution of complex alterations to core-binding motifs. Another limitation is the nature of the zinc finger, which can regulate different genes in the presence of a co-factor. This different regulation mechanism increases the analysis difficulty since other types of transcription factor can bind to different sequences making the “predictive” approach useless. Vital information can be obtained using an immune precipitation approach to identify other proteins with which a protein can collaborate. Furthermore, given the variety of pathways that CRZ1 regulates, it is necessary to conduct a multi-omics analysis combined with transcriptome sequencing to understand the full metabolic pathway in fungi.
Data availability
No datasets were generated or analysed during the current study.
References
Hsu IS, Strome B, Plotnikov S, Moses AM (2019) ‘A Noisy Analog-to-Digital Converter Connects Cytosolic Calcium Bursts to Transcription Factor Nuclear Localization Pulses in Yeast’, G3 GenesGenomesGenetics, vol. 9, no. 2, pp. 561–570, Feb. https://doi.org/10.1534/g3.118.200841
Sneyd J et al (2017) Feb., ‘On the dynamical structure of calcium oscillations’, Proc. Natl. Acad. Sci, vol. 114, no. 7, pp. 1456–1461, https://doi.org/10.1073/pnas.1614613114
Zhao Y, Du J, Zhao G, Jiang L (2013) ‘Activation of calcineurin is mainly responsible for the calcium sensitivity of gene deletion mutations in the genome of budding yeast’, Genomics, vol. 101, no. 1, pp. 49–56, Jan. https://doi.org/10.1016/j.ygeno.2012.09.005
Thewes S (2014) Calcineurin-Crz1 signaling in Lower eukaryotes. Eukaryot Cell 13:694–705. https://doi.org/10.1128/EC.00038-14
Yang Y, Xie P, Li Y, Bi Y, Prusky DB (2022) ‘Updating Insights into the Regulatory Mechanisms of Calcineurin-Activated Transcription Factor Crz1 in Pathogenic Fungi’, J. Fungi, vol. 8, no. 10, Art. no. 10, Oct. https://doi.org/10.3390/jof8101082
Razin SV, Borunova VV, Maksimenko OG, Kantidze OL (2012) ‘Cys2His2 zinc finger protein family: Classification, functions, and major members’, Biochem. Mosc, vol. 77, no. 3, pp. 217–226, Mar. https://doi.org/10.1134/S0006297912030017
MacPherson S, Larochelle M, Turcotte B (2006) ‘A Fungal Family of Transcriptional Regulators: the Zinc Cluster Proteins’, Microbiol. Mol. Biol. Rev, vol. 70, no. 3, pp. 583–604, Sep. https://doi.org/10.1128/MMBR.00015-06
Laity JH, Lee BM, Wright PE (Feb. 2001) Zinc finger proteins: new insights into structural and functional diversity. Curr Opin Struct Biol 11(1):39–46. https://doi.org/10.1016/S0959-440X(00)00167-6
Shelest E (May 2017) Transcription factors in Fungi: TFome dynamics, three major families, and dual-specificity TFs. Front Genet 8:53. https://doi.org/10.3389/fgene.2017.00053
Johnston M (1987) ‘A model fungal gene regulatory mechanism: the GAL genes of Saccharomyces cerevisiae’. MICROBIOL REV, 51
Schjerling P (1996) ‘Comparative amino acid sequence analysis of the C6 zinc cluster family of transcriptional regulators’, Nucleic Acids Res, vol. 24, no. 23, pp. 4599–4607, Dec. https://doi.org/10.1093/nar/24.23.4599
Mamane Y, Hellauer K, Rochon M-H, Turcotte B (1998) ‘A Linker Region of the Yeast Zinc Cluster Protein Leu3p Specifies Binding to Everted Repeat DNA’, J. Biol. Chem, vol. 273, no. 29, pp. 18556–18561, Jul. https://doi.org/10.1074/jbc.273.29.18556
Todd RB, Andrianopoulos A (1997) ‘Evolution of a Fungal Regulatory Gene Family: The Zn(II)2Cys6 Binuclear Cluster DNA Binding Motif’, Fungal Genet. Biol, vol. 21, no. 3, pp. 388–405, Jun. https://doi.org/10.1006/fgbi.1997.0993
Chang P-K, Ehrlich KC (May 2013) Genome-wide analysis of the zn(II)2Cys6 zinc cluster-encoding gene family in aspergillus flavus. Appl Microbiol Biotechnol 97(10):4289–4300. https://doi.org/10.1007/s00253-013-4865-2
Cyert MS (Nov. 2003) Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem Biophys Res Commun 311(4):1143–1150. https://doi.org/10.1016/S0006-291X(03)01552-3
Yoshimoto H et al (2002) Aug., ‘Genome-wide Analysis of Gene Expression Regulated by the Calcineurin/Crz1p Signaling Pathway in Saccharomyces cerevisiae’, J. Biol. Chem, vol. 277, no. 34, pp. 31079–31088, https://doi.org/10.1074/jbc.M202718200
Martins-Santana L, de Paula RG, Silva AG, Lopes DCB, Silva R, Silva-Rocha R (2020) ‘CRZ1 regulator and calcium cooperatively modulate holocellulases gene expression in Trichoderma reesei QM6a’, Genet. Mol. Biol, vol. 43, no. 2, p. e20190244, https://doi.org/10.1590/1678-4685-gmb-2019-0244
Xu H, Fang T, Omran RP, Whiteway M, Jiang L (2020) ‘RNA sequencing reveals an additional Crz1-binding motif in promoters of its target genes in the human fungal pathogen Candida albicans’, Cell Commun. Signal, vol. 18, no. 1, p. 1, Dec. https://doi.org/10.1186/s12964-019-0473-9
Noël J, Turcotte B (1998) ‘Zinc Cluster Proteins Leu3p and Uga3p Recognize Highly Related but Distinct DNA Targets’, J. Biol. Chem, vol. 273, no. 28, pp. 17463–17468, Jul. https://doi.org/10.1074/jbc.273.28.17463
Jungwirth H, Kuchler K (Feb. 2006) Yeast ABC transporters - a tale of sex, stress, drugs and aging. FEBS Lett 580(4):1131–1138. https://doi.org/10.1016/j.febslet.2005.12.050
Rudolph HK et al (1989) Jul., ‘The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2 + ATPase family’, Cell, vol. 58, no. 1, pp. 133–145, https://doi.org/10.1016/0092-8674(89)90410-8
Huh W-K et al (2003) Oct., ‘Global analysis of protein localization in budding yeast’, Nature, vol. 425, no. 6959, pp. 686–691, https://doi.org/10.1038/nature02026
Spielvogel A et al (2008) Two zinc finger transcription factors, CrzA and SltA, are involved in cation homoeostasis and detoxification in aspergillus nidulans. Biochem J 414 3:419–429. https://doi.org/10.1042/BJ20080344
Zhao K et al (Jan. 2022) Drafting Penicillium Oxalicum calcineurin-CrzA pathway by combining the analysis of phenotype, transcriptome, and endogenous protein–protein interactions. Fungal Genet Biol 158:103652. https://doi.org/10.1016/j.fgb.2021.103652
Liu S, Hou Y, Liu W, Lu C, Wang W, Sun S (2015) Components of the calcium-calcineurin signaling pathway in fungal cells and their potential as antifungal targets. Eukaryot Cell 14:324–334. https://doi.org/10.1128/EC.00271-14
Tisi R, Rigamonti M, Groppi S, Belotti F ‘Calcium homeostasis and signaling in fungi and their relevance for pathogenicity of yeasts and filamentous fungi’, 2016, https://doi.org/10.3934/MOLSCI.2016.4.505
Cronin SR, Rao R, Hampton RY (2002) ‘Cod1p/Spf1p is a P-type ATPase involved in ER function and Ca2 + homeostasis’, J. Cell Biol, vol. 157, no. 6, pp. 1017–1028, Jun. https://doi.org/10.1083/jcb.200203052
Goldman A et al (Aug. 2014) The Calcineurin Signaling Network Evolves via Conserved Kinase-Phosphatase Modules that transcend substrate identity. Mol Cell 55(3):422–435. https://doi.org/10.1016/j.molcel.2014.05.012
Juvvadi P, Lee SC, Heitman J, Steinbach W (2017) ‘Calcineurin in fungal virulence and drug resistance: Prospects for harnessing targeted inhibition of calcineurin for an antifungal therapeutic approach’, Virulence, vol. 8, pp. 186–197, https://doi.org/10.1080/21505594.2016.1201250
Carbó N, Tarkowski N, Ipiña EP, Dawson SP, Aguilar PS (Feb. 2017) Sexual pheromone modulates the frequency of cytosolic ca 2+ bursts in Saccharomyces cerevisiae. Mol Biol Cell 28(4):501–510. https://doi.org/10.1091/mbc.e16-07-0481
Cui J, Kaandorp J, Sloot P, Lloyd C, Filatov M (2009) Calcium homeostasis and signaling in yeast cells and cardiac myocytes. FEMS Yeast Res 9 8:1137–1147. https://doi.org/10.1111/j.1567-1364.2009.00552.x
Cai L, Dalal CK, Elowitz MB (2008) ‘Frequency-modulated nuclear localization bursts coordinate gene regulation’, Nature, vol. 455, no. 7212, pp. 485–490, Sep. https://doi.org/10.1038/nature07292
Cui J, Kaandorp JA (2006) ‘Mathematical modeling of calcium homeostasis in yeast cells’, Cell Calcium, vol. 39, no. 4, pp. 337–348, Apr. https://doi.org/10.1016/j.ceca.2005.12.001
Dalal CK, Cai L, Lin Y, Rahbar K, Elowitz MB (Sep. 2014) Pulsatile dynamics in the yeast proteome. Curr Biol 24:2189–2194. https://doi.org/10.1016/j.cub.2014.07.076
Levine JH, Lin Y, Elowitz MB (2013) ‘Functional Roles of Pulsing in Genetic Circuits’, Science, vol. 342, no. 6163, pp. 1193–1200, Dec. https://doi.org/10.1126/science.1239999
Karababa M, Valentino E, Pardini G, Coste A, Billé J, Sanglard D (2006) ‘CRZ1, a target of the calcineurin pathway in Candida albicans’, Mol. Microbiol, vol. 59, p. null, https://doi.org/10.1111/j.1365-2958.2005.05037.x
Boustany LM, Cyert MS (2002) ‘Calcineurin-dependent regulation of Crz1p nuclear export requires Msn5p and a conserved calcineurin docking site’, Genes Dev, vol. 16, no. 5, pp. 608–619, Mar. https://doi.org/10.1101/gad.967602
Polizotto RS, Cyert MS (2001) ‘Calcineurin-dependent nuclear import of the transcription factor Crz1p requires Nmd5p’, J. Cell Biol, vol. 154, no. 5, pp. 951–960, Sep. https://doi.org/10.1083/jcb.200104078
Chen Y-L et al (2012) Convergent evolution of Calcineurin Pathway Roles in Thermotolerance and Virulence in Candida Glabrata’, G3 GenesGenomesGenetics. Jun 2(6):675–691. https://doi.org/10.1534/g3.112.002279
Lev S, Desmarini D, Chayakulkeeree M, Sorrell T, Djordjevic J (2012) ‘The Crz1/Sp1 Transcription Factor of Cryptococcus neoformans Is Activated by Calcineurin and Regulates Cell Wall Integrity’, PLoS ONE, vol. 7, p. null, https://doi.org/10.1371/journal.pone.0051403
Espeso E (2016) The CRaZy calcium cycle. Adv Exp Med Biol 892:169–186. https://doi.org/10.1007/978-3-319-25304-6_7
Schumacher J, de Larrinoa ID, Tudzynski B (2008) Calcineurin-responsive zinc finger transcription factor CRZ1 of Botrytis cinerea is required for growth, Development, and full virulence on Bean plants. Eukaryot Cell 7:584–601. https://doi.org/10.1128/EC.00426-07
Hagiwara D, Kondo A, Fujioka T, Abe K (2008) ‘Functional analysis of C2H2 zinc finger transcription factor CrzA involved in calcium signaling in Aspergillus nidulans’, Curr. Genet, vol. 54, no. 6, pp. 325–338, Dec. https://doi.org/10.1007/s00294-008-0220-z
Chen L, Tong Q, Zhang C, Ding K (Feb. 2019) The transcription factor FgCrz1A is essential for fungal development, virulence, deoxynivalenol biosynthesis and stress responses in Fusarium Graminearum. Curr Genet 65(1):153–166. https://doi.org/10.1007/s00294-018-0853-5
Gao L et al (Nov. 2011) Osmotic stabilizer-coupled suppression of NDR defects is dependent on the calcium–calcineurin signaling cascade in aspergillus nidulans. Cell Signal 23(11):1750–1757. https://doi.org/10.1016/j.cellsig.2011.06.009
Wang Y, Wang Y, Tian C (2013) ‘Quantitative Detection of Pathogen DNA of Verticillium Wilt on Smoke Tree Cotinus coggygria’, Plant Dis, vol. 97, no. 12, pp. 1645–1651, Dec. https://doi.org/10.1094/PDIS-04-13-0406-RE
Xiong D, Wang Y, Tang C, Fang Y, Zou J-M, Tian C (2015) VdCrz1 is involved in microsclerotia formation and required for full virulence in Verticillium Dahliae. Fungal Genet Biol FG B 82:201–212. https://doi.org/10.1016/j.fgb.2015.07.011
Klimes A, Dobinson KF (2006) ‘A hydrophobin gene, VDH1, is involved in microsclerotial development and spore viability in the plant pathogen Verticillium dahliae’, Fungal Genet. Biol, vol. 43, no. 4, pp. 283–294, Apr. https://doi.org/10.1016/j.fgb.2005.12.006
Cunningham K, Fink G (Feb. 1994) Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2 + ATPases. J Cell Biol 124(3):351–363. https://doi.org/10.1083/jcb.124.3.351
Wang K-D, Borrego EJ, Kenerley CM, Kolomiets MV (2020) ‘Oxylipins Other Than Jasmonic Acid Are Xylem-Resident Signals Regulating Systemic Resistance Induced by Trichoderma virens in Maize’, Plant Cell, vol. 32, no. 1, pp. 166–185, Jan. https://doi.org/10.1105/tpc.19.00487
He F et al (Jun. 2016) The transcription factor VpCRZ1 is required for fruiting body formation and pathogenicity in Valsa pyri. Microb Pathog 95:101–110. https://doi.org/10.1016/j.micpath.2016.02.018
Leplat J, Friberg H, Abid M, Steinberg C (Jan. 2013) Survival of Fusarium Graminearum, the causal agent of Fusarium head blight. A review. Agron Sustain Dev 33(1):97–111. https://doi.org/10.1007/s13593-012-0098-5
Xu M, Wang Q, Wang G, Zhang X, Liu H, Jiang C (Oct. 2022) Combatting Fusarium head blight: advances in molecular interactions between Fusarium graminearum and wheat. Phytopathol Res 4(1):37. https://doi.org/10.1186/s42483-022-00142-0
Choi J, Kim Y-S, Kim S, Park J, Lee Y-H (2009) MoCRZ1, a gene encoding a calcineurin-responsive transcription factor, regulates fungal growth and pathogenicity of Magnaporthe oryzae. Fungal Genet Biol FG B 46 3:243–254. https://doi.org/10.1016/j.fgb.2008.11.010
Zhang H, Zhao Q, Liu K, Zhang Z, Wang Y, Zheng X (2009) MgCRZ1, a transcription factor of Magnaporthe Grisea, controls growth, development and is involved in full virulence. FEMS Microbiol Lett 293(2):160–169. https://doi.org/10.1111/j.1574-6968.2009.01524.x
Chen X, Liu Y, Keyhani NO, Xia Y, Cao Y (2017) ‘The regulatory role of the transcription factor Crz1 in stress tolerance, pathogenicity, and its target gene expression in Metarhizium acridum’, Appl. Microbiol. Biotechnol, vol. 101, no. 12, pp. 5033–5043, Jun. https://doi.org/10.1007/s00253-017-8290-9
Zhang T, Xu Q, Sun X, Li H (2013) The calcineurin-responsive transcription factor Crz1 is required for conidation, full virulence and DMI resistance in Penicillium Digitatum. Microbiol Res 168(4):211–222. https://doi.org/10.1016/j.micres.2012.11.006
Roque A, Petrezsélyová S, Serra-Cardona A, Ariño J (Dec. 2016) Genome-wide recruitment profiling of transcription factor Crz1 in response to high pH stress. BMC Genomics 17(1):662. https://doi.org/10.1186/s12864-016-3006-6
Zhang J et al (2012) Aug., ‘Calcineurin Is Required for Pseudohyphal Growth, Virulence, and Drug Resistance in Candida lusitaniae’, PLoS ONE, vol. 7, no. 8, p. e44192, https://doi.org/10.1371/journal.pone.0044192
Finotti E et al (Aug. 2021) Aflatoxins are natural scavengers of reactive oxygen species. Sci Rep 11(1):16024. https://doi.org/10.1038/s41598-021-95325-8
Mudge AM, Dill-Macky R, Dong Y, Gardiner DM, White RG, Manners JM (2006) ‘A role for the mycotoxin deoxynivalenol in stem colonisation during crown rot disease of wheat caused by Fusarium graminearum and Fusarium pseudograminearum’, Physiol. Mol. Plant Pathol, vol. 69, no. 1–3, pp. 73–85, Jul. https://doi.org/10.1016/j.pmpp.2007.01.003
Lim S-Y, Son Y-E, Lee D-H, Eom T-J, Kim M-J, Park H-S (2019) ‘Function of crzA in Fungal Development and Aflatoxin Production in Aspergillus flavus’, Toxins, vol. 11, no. 10, p. 567, Sep. https://doi.org/10.3390/toxins11100567
Acknowledgements
None.
Funding
No funding.
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
MR contributed to conceive the study. The first draft of manuscript was written by AC, MB and MR supervised and reviewed the manuscript. AC and MB prepared the figures. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
It is not applicable.
Consent to participate
It is not applicable.
Consent for publication
It is not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cacciotti, A., Beccaccioli, M. & Reverberi, M. The CRZ1 transcription factor in plant fungi: regulation mechanism and impact on pathogenesis. Mol Biol Rep 51, 647 (2024). https://doi.org/10.1007/s11033-024-09593-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09593-4