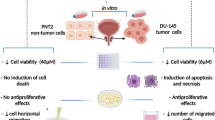
Abstract
Prostate cancer is a malignant epithelial tumor of the prostate gland and is the most common malignant tumor of the male genitourinary system. Pharmacological therapies, including chemotherapy and androgen deprivation therapy, play a key role in the treatment of prostate cancer. However, drug resistance and side effects limit the use of these drugs and so there is a need for new drug therapies for prostate cancer patients. Flavonoids, with their wide range of sources and diverse biological activities, have attracted much attention in the field of anti-tumor drug screening. In 2016, at least 58 flavonoids were reported to have anti-prostate cancer activity. In recent years, six additional flavonoid compounds have been found to have anti-prostate cancer potential. In this review, we have collected a large amount of evidence on the anti-prostate cancer effects of these six flavonoids, including a large number of cellular experiments and a small number of preclinical animal experiments. In addition, we predicted their drug-forming properties using Schrödinger’s QikProp software and ADMETlab due to the lack of in vivo pharmacokinetic data for the six compounds. In conclusion, this review has fully confirmed the anti-prostate cancer effects of these six flavonoids, summarized their mechanisms of action and predicted their druggability. It provides a reference for the further development of these compounds into anti-prostate cancer drugs.


Similar content being viewed by others
Data availability
Not applicable.
References
Siegel RL, Miller KD, Wagle NS et al (2023) Cancer statistics, 2023. CA Cancer J Clin 73:17–48. https://doi.org/10.3322/caac.21763
Pagliuca M, Buonerba C, Fizazi K et al (2019) The evolving systemic treatment landscape for patients with advanced prostate cancer. Drugs 79:381–400. https://doi.org/10.1007/s40265-019-1060-5
Evans AJ (2018) Treatment effects in prostate cancer. Mod Pathol 31:S110-121. https://doi.org/10.1038/modpathol.2017.158
Nader R, El Amm J, Aragon-Ching JB (2018) Role of chemotherapy in prostate cancer. Asian J Androl 20:221–229. https://doi.org/10.4103/aja.aja_40_17
Zraik IM, Heß-Busch Y (2021) Management of chemotherapy side effects and their long-term sequelae. Urologe A 60:862–871. https://doi.org/10.1007/s00120-021-01569-7
Bilusic M, Madan RA, Gulley JL (2017) Immunotherapy of prostate cancer: facts and hopes. Clin Cancer Res 23:6764–6770. https://doi.org/10.1158/1078-0432.Ccr-17-0019
De Velasco MA, Uemura H (2018) Prostate cancer immunotherapy: where are we and where are we going? Curr Opin Urol 28:15–24. https://doi.org/10.1097/mou.0000000000000462
Gheorghe GS, Hodorogea AS, Ciobanu A et al (2021) Androgen deprivation therapy, hypogonadism and cardiovascular toxicity in men with advanced prostate cancer. Curr Oncol 28:3331–3346. https://doi.org/10.3390/curroncol28050289
Gómez-Aparicio MA, López-Campos F, Pelari-Mici L et al (2022) Bone health and therapeutic agents in advanced prostate cancer. Front Biosci (Landmark Ed) 27:34. https://doi.org/10.31083/j.fbl2701034
Wen K, Fang X, Yang J et al (2021) Recent research on flavonoids and their biomedical applications. Curr Med Chem 28:1042–1066. https://doi.org/10.2174/0929867327666200713184138
Kopustinskiene DM, Jakstas V, Savickas A et al (2020) Flavonoids as anticancer agents. Nutrients. https://doi.org/10.3390/nu12020457
Vue B, Zhang S, Chen QH (2016) Flavonoids with therapeutic potential in prostate cancer. Anticancer Agents Med Chem 16:1205–1229. https://doi.org/10.2174/1871520615666151008122622
Flaig TW, Gustafson DL, Su LJ et al (2007) A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs 25:139–146. https://doi.org/10.1007/s10637-006-9019-2
Flaig TW, Glodé M, Gustafson D et al (2010) A study of high-dose oral silybin-phytosome followed by prostatectomy in patients with localized prostate cancer. Prostate 70:848–855. https://doi.org/10.1002/pros.21118
Nguyen MM, Ahmann FR, Nagle RB et al (2012) Randomized, double-blind, placebo-controlled trial of polyphenon E in prostate cancer patients before prostatectomy: evaluation of potential chemopreventive activities. Cancer Prev Res (Phila) 5:290–298. https://doi.org/10.1158/1940-6207.Capr-11-0306
McLarty J, Bigelow RL, Smith M et al (2009) Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev Res (Phila) 2:673–682. https://doi.org/10.1158/1940-6207.Capr-08-0167
Liu W, Feng Y, Yu S et al (2021) The flavonoid biosynthesis network in plants. Int J Mol Sci. https://doi.org/10.3390/ijms222312824
Varughese RS, Lam WS, Marican A et al (2019) Biopharmacological considerations for accelerating drug development of deguelin, a rotenoid with potent chemotherapeutic and chemopreventive potential. Cancer 125:1789–1798. https://doi.org/10.1002/cncr.32069
Zhang P, Zhang M, Mellich TA et al (2022) Variation in rotenone and deguelin contents among strains across four tephrosia species and their activities against aphids and whiteflies. Toxins (Basel). https://doi.org/10.3390/toxins14050339
Thamilselvan V, Menon M, Thamilselvan S (2011) Anticancer efficacy of deguelin in human prostate cancer cells targeting glycogen synthase kinase-3 β/β-catenin pathway. Int J Cancer 129:2916–2927. https://doi.org/10.1002/ijc.25949
Adnan M, Rasul A, Hussain G et al (2020) Ginkgetin: a natural biflavone with versatile pharmacological activities. Food Chem Toxicol 145:111642. https://doi.org/10.1016/j.fct.2020.111642
Jeon YJ, Jung SN, Yun J et al (2015) Ginkgetin inhibits the growth of DU-145 prostate cancer cells through inhibition of signal transducer and activator of transcription 3 activity. Cancer Sci 106:413–420. https://doi.org/10.1111/cas.12608
You OH, Kim SH, Kim B et al (2013) Ginkgetin induces apoptosis via activation of caspase and inhibition of survival genes in PC-3 prostate cancer cells. Bioorg Med Chem Lett 23:2692–2695. https://doi.org/10.1016/j.bmcl.2013.02.080
Gong G, Guan YY, Zhang ZL et al (2020) Isorhamnetin: a review of pharmacological effects. Biomed Pharmacother 128:110301. https://doi.org/10.1016/j.biopha.2020.110301
Cai F, Zhang Y, Li J et al (2020) Isorhamnetin inhibited the proliferation and metastasis of androgen-independent prostate cancer cells by targeting the mitochondrion-dependent intrinsic apoptotic and PI3K/Akt/mTOR pathway. Biosci Rep. https://doi.org/10.1042/bsr20192826
Ghanbari-Movahed M, Shafiee S, Burcher JT et al (2023) Anticancer potential of apigenin and isovitexin with focus on oncogenic metabolism in cancer stem cells. Metabolites. https://doi.org/10.3390/metabo13030404
He M, Min JW, Kong WL et al (2016) A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 115:74–85. https://doi.org/10.1016/j.fitote.2016.09.011
Hanafi MMM, Afzan A, Yaakob H et al (2017) In vitro pro-apoptotic and anti-migratory effects of Ficus deltoidea L. plant extracts on the human prostate cancer cell lines PC3. Front Pharmacol 8:895. https://doi.org/10.3389/fphar.2017.00895
Noguchi S, Atsumi H, Iwao Y et al (2016) Nobiletin: a citrus flavonoid displaying potent physiological activity. Acta Crystallogr C Struct Chem 72:124–127. https://doi.org/10.1107/s2053229616000577
Chen J, Creed A, Chen AY et al (2014) Nobiletin suppresses cell viability through AKT pathways in PC-3 and DU-145 prostate cancer cells. BMC Pharmacol Toxicol 15:59. https://doi.org/10.1186/2050-6511-15-59
Deveci Ozkan A, Kaleli S, Onen HI et al (2020) Anti-inflammatory effects of nobiletin on TLR4/TRIF/IRF3 and TLR9/IRF7 signaling pathways in prostate cancer cells. Immunopharmacol Immunotoxicol 42:93–100. https://doi.org/10.1080/08923973.2020.1725040
Liu Y, Yu C, Shao Z et al (2021) Selective degradation of AR-V7 to overcome castration resistance of prostate cancer. Cell Death Dis 12:857. https://doi.org/10.1038/s41419-021-04162-0
Ozkan AD, Kaleli SM, S, (2021) Evaluation of the effects of nobiletin on toll-like receptor 3 signaling pathways in prostate cancer in vitro. Nutr Cancer 73:1138–1144. https://doi.org/10.1080/01635581.2020.1841247
Ma Y, Ren X, Patel N et al (2020) Nobiletin, a citrus polymethoxyflavone, enhances the effects of bicalutamide on prostate cancer cells via down regulation of NF-κB, STAT3, and ERK activation. RSC Adv 10:10254–10262. https://doi.org/10.1039/c9ra10020b
Tang M, Ogawa K, Asamoto M et al (2007) Protective effects of citrus nobiletin and auraptene in transgenic rats developing adenocarcinoma of the prostate (TRAP) and human prostate carcinoma cells. Cancer Sci 98:471–477. https://doi.org/10.1111/j.1349-7006.2007.00417.x
Tang MX, Ogawa K, Asamoto M et al (2011) Effects of nobiletin on PhIP-induced prostate and colon carcinogenesis in F344 rats. Nutr Cancer 63:227–233. https://doi.org/10.1080/01635581.2011.523506
Guney Eskiler G, Deveci AO, Bilir C et al (2019) Synergistic effects of nobiletin and sorafenib combination on metastatic prostate cancer cells. Nutr Cancer 71:1299–1312. https://doi.org/10.1080/01635581.2019.1601237
Tuli HS, Rath P, Chauhan A et al (2022) Phloretin, as a potent anticancer compound: from chemistry to cellular interactions. Molecules. https://doi.org/10.3390/molecules27248819
Nakhate KT, Badwaik H, Choudhary R et al (2022) Therapeutic potential and pharmaceutical development of a multitargeted flavonoid phloretin. Nutrients. https://doi.org/10.3390/nu14173638
Gonzalez-Menendez P, Hevia D, Rodriguez-Garcia A et al (2014) Regulation of GLUT transporters by flavonoids in androgen-sensitive and -insensitive prostate cancer cells. Endocrinology 155:3238–3250. https://doi.org/10.1210/en.2014-1260
Kim U, Kim CY, Lee JM et al (2020) Correction to: phloretin inhibits the human prostate cancer cells through the generation of reactive oxygen species. Pathol Oncol Res 26:2011–2012. https://doi.org/10.1007/s12253-019-00667-4
Kang D, Zuo W, Wu Q et al (2020) Inhibition of specificity protein 1 is involved in phloretin-induced suppression of prostate cancer. Biomed Res Int 2020:1358674. https://doi.org/10.1155/2020/1358674
Vissenaekens H, Criel H, Grootaert C et al (2022) Flavonoids and cellular stress: a complex interplay affecting human health. Crit Rev Food Sci Nutr 62:8535–8566. https://doi.org/10.1080/10408398.2021.1929822
Cui Z, Zhao X, Amevor FK et al (2022) Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front Immunol 13:943321. https://doi.org/10.3389/fimmu.2022.943321
Verdoorn BP, Evans TK, Hanson GJ et al (2021) Fisetin for COVID-19 in skilled nursing facilities: senolytic trials in the COVID era. J Am Geriatr Soc 69:3023–3033. https://doi.org/10.1111/jgs.17416
Benavente-García O, Castillo J, Del Baño MJ et al (2001) Improved water solubility of neohesperidin dihydrochalcone in sweetener blends. J Agric Food Chem 49:189–191. https://doi.org/10.1021/jf000186l
Ganesan P, Choi DK (2016) Current application of phytocompound-based nanocosmeceuticals for beauty and skin therapy. Int J Nanomedicine 11:1987–2007. https://doi.org/10.2147/ijn.S104701
Mamouni K, Zhang S, Li X et al (2018) A novel flavonoid composition targets androgen receptor signaling and inhibits prostate cancer growth in preclinical models. Neoplasia 20:789–799. https://doi.org/10.1016/j.neo.2018.06.003
Mukhtar E, Adhami VM, Siddiqui IA et al (2016) Fisetin enhances chemotherapeutic effect of cabazitaxel against human prostate cancer cells. Mol Cancer Ther 15:2863–2874. https://doi.org/10.1158/1535-7163.Mct-16-0515
Yang F, Song L, Wang H et al (2015) Combination of quercetin and 2-methoxyestradiol enhances inhibition of human prostate cancer LNCaP and PC-3 cells xenograft tumor growth. PLoS ONE 10:e0128277. https://doi.org/10.1371/journal.pone.0128277
Caminero Gomes Soares A, Marques Sousa GH, Calil RL et al (2023) absorption matters: a closer look at popular oral bioavailability rules for drug approvals. Mol Inform 42:e202300115. https://doi.org/10.1002/minf.202300115
Acknowledgements
None.
Funding
This research was funded by the Specific Research Project of Guangxi for Research Bases and Talents (AD20159033); and the Initial Scientific Research Fund of Guangxi University of Chinese Medicine (2017BS037).
Author information
Authors and Affiliations
Contributions
Conceptualization, X.W.; methodology, X.W.; software, X.W.; formal analysis, X.W.; investigation, W.F., J.D. and M.N; resources, X.W. and J.D.; data curation, W.F. and J.D.; writing—original draft preparation, W.F. and J.D.; writing—review and editing, X.W.; visualization, W.F. and X.W.; funding acquisition, J.D. and X.W.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
Not applicable.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fang, W., Du, J., Nie, M. et al. Recent advances in flavonoid compounds for the treatment of prostate cancer. Mol Biol Rep 51, 653 (2024). https://doi.org/10.1007/s11033-024-09567-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09567-6