Abstract
Background
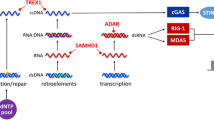
Type I interferons (IFNs) are an essential class of cytokines with antitumor, antiviral and immunoregulatory effects. However, over-productive the type I IFNs are tightly associated with autoimmune disorders. Thus, the induction of type I interferons is precisely regulated to maintain immune hemostasis. This study aimed to identify a novel regulator of type I interferon signaling.
Methods and results
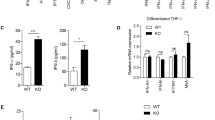
Primary BMDMs, isolated from mice, and human cell lines (HEK293 cells, Hela cells) and murine cell line (MEF cells) were cultured to generate in vitro models. After knockdown VRK1, real-time PCR and dual-luciferase reporter assay were performed to determine the expression level of the type I IFNs and ISGs following HTDNA and Poly (dA:dT) stimulation. Additionally, cells were treated with the VRK1 inhibitor, and the impact of VRK1 inhibition was detected. Upon HTDNA and Poly (dA:dT) stimulation, knockdown of VRK1 attenuated the induction of the type I IFNs and ISGs. Consistently, VRK-IN-1, a potent and selective VRK1 inhibitor, significantly suppressed the induction of the type I IFNs and ISGs in human and murine cell lines. Further, VRK-IN-1 inhibited induction of the type I IFNs in mouse primary BMDMs. Intriguingly, VRK1 potentiated the cGAS-STING- IFN-I axis response at STING level.
Conclusions
Our study reveals a novel function of VRK1 in regulating the production of type I IFNs. VRK-IN-1 might be a potential lead compound for suppressing aberrant type I IFNs in autoimmune disorders.






Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- PRRs:
-
Pattern recognition receptors
- PAMPs:
-
Pathogen-associated molecular patterns
- VRKs:
-
Vaccinia-Related Kinases
- BMDMs:
-
Bone marrow-derived macrophages
- cGAS:
-
Cytosolic GAMP synthase
- TBK1:
-
TANK-binding kinase 1
- RIG-I:
-
RNA helicases retinoic acid-inducible gene I
- MDA5:
-
Melanoma differentiation-associated gene 5
- DAI:
-
DNA-dependent activator of IFN-regulatory factors
- AIM2:
-
Absent in melanoma 2
References
Capobianchi MR et al (2015) Type I IFN family members: similarity, differences and interaction. Cytokine Growth Factor Rev 26(2):103–111. https://doi.org/10.1016/j.cytogfr.2014.10.011
Kaur BP, Secord E (2021) Innate immunity. Immunol Allergy Clin North Am 41(4):535–541. https://doi.org/10.1016/j.iac.2021.07.003
Barton GM, Medzhitov R (2002) Toll-like receptors and their ligands. Curr Top Microbiol Immunol 270:81–92. https://doi.org/10.1007/978-3-642-59430-4_5
Thoresen D et al (2021) The molecular mechanism of RIG-I activation and signaling. Immunol Rev 304(1):154–168. https://doi.org/10.1111/imr.13022
Takaoka A et al (2007) DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448(7152):501–505. https://doi.org/10.1038/nature06013
Barber GN (2011) Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses. Curr Opin Immunol 23(1):10–20. https://doi.org/10.1016/j.coi.2010.12.015
Sun L et al (2013) Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339(6121):786–791. https://doi.org/10.1126/science.1232458
Kumagai Y, Takeuchi O, Akira S (2008) TLR9 as a key receptor for the recognition of DNA. Adv Drug Deliv Rev 60(7):795–804. https://doi.org/10.1016/j.addr.2007.12.004
Ahlers LR, Goodman AG (2016) Nucleic acid sensing and innate immunity: signaling pathways controlling viral pathogenesis and autoimmunity. Curr Clin Microbiol Rep 3(3):132–141. https://doi.org/10.1007/s40588-016-0043-5
Chen C, Xu P (2023) Cellular functions of cGAS-STING signaling. Trends Cell Biol 33(8):630–648. https://doi.org/10.1016/j.tcb.2022.11.001
Decout A et al (2021) The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol 21(9):548–569. https://doi.org/10.1038/s41577-021-00524-z
Kwon J, Bakhoum SF (2020) The cytosolic DNA-Sensing cGAS-STING pathway in Cancer. Cancer Discov 10(1):26–39. https://doi.org/10.1158/2159-8290.Cd-19-0761
Pan M et al (2023) UXT attenuates the CGAS-STING1 signaling by targeting STING1 for autophagic degradation. Autophagy 19(2):440–456. https://doi.org/10.1080/15548627.2022.2076192
Pan M et al (2024) CSNK1A1/CK1α suppresses autoimmunity by restraining the CGAS-STING1 signaling. Autophagy 20(2):311–328. https://doi.org/10.1080/15548627.2023.2256135
Hu Y et al (2022) Emerging role of the cGAS-STING signaling pathway in autoimmune diseases: biologic function, mechanisms and clinical prospection. Autoimmun Rev 21(9):103155. https://doi.org/10.1016/j.autrev.2022.103155
Skopelja-Gardner S, An J, Elkon KB (2022) Role of the cGAS-STING pathway in systemic and organ-specific diseases. Nat Rev Nephrol 18(9):558–572. https://doi.org/10.1038/s41581-022-00589-6
Klerkx EP, Lazo PA, Askjaer P (2009) Emerging biological functions of the vaccinia-related kinase (VRK) family. Histol Histopathol 24(6):749–759. https://doi.org/10.14670/hh-24.749
Manning G et al (2002) The protein kinase complement of the human genome. Science 298(5600):1912–1934. https://doi.org/10.1126/science.1075762
Valbuena A, López-Sánchez I, Lazo PA (2008) Human VRK1 is an early response gene and its loss causes a block in cell cycle progression. PLoS ONE 3(2):e1642. https://doi.org/10.1371/journal.pone.0001642
Du N, Zhang B, Zhang Y (2023) Downregulation of VRK1 inhibits progression of lung squamous cell carcinoma through DNA damage. Can Respir J 2023(p 4533504). https://doi.org/10.1155/2023/4533504
Chang X et al (2023) Downregulating vaccinia-related kinase 1 by luteolin suppresses ovarian cancer cell proliferation by activating the p53 signaling pathway. Gynecol Oncol 173:31–40. https://doi.org/10.1016/j.ygyno.2023.04.003
Shields JA et al (2022) VRK1 is a synthetic-Lethal Target in VRK2-Deficient glioblastoma. Cancer Res 82(21):4044–4057. https://doi.org/10.1158/0008-5472.Can-21-4443
So J et al (2022) VRK1 as a synthetic lethal target in VRK2 promoter-methylated cancers of the nervous system. JCI Insight 7(19). https://doi.org/10.1172/jci.insight.158755
Nichols RJ, Wiebe MS, Traktman P (2006) The vaccinia-related kinases phosphorylate the N’ terminus of BAF, regulating its interaction with DNA and its retention in the nucleus. Mol Biol Cell 17(5):2451–2464. https://doi.org/10.1091/mbc.e05-12-1179
Serafim RAM et al (2019) Development of pyridine-based inhibitors for the human vaccinia-related kinases 1 and 2. ACS Med Chem Lett 10(9):1266–1271. https://doi.org/10.1021/acsmedchemlett.9b00082
Weischenfeldt J, Porse B, Protoc CSH (2008) 2008: p. pdb.prot5080. https://doi.org/10.1101/pdb.prot5080
Zheng W et al (2021) How the Innate Immune DNA sensing cGAS-STING pathway is involved in Autophagy. Int J Mol Sci 22(24). https://doi.org/10.3390/ijms222413232
Kang TH et al (2007) Mitotic histone H3 phosphorylation by vaccinia-related kinase 1 in mammalian cells. Mol Cell Biol 27(24):8533–8546. https://doi.org/10.1128/mcb.00018-07
Sevilla A et al (2004) Human vaccinia-related kinase 1 (VRK1) activates the ATF2 transcriptional activity by novel phosphorylation on Thr-73 and Ser-62 and cooperates with JNK. J Biol Chem 279(26):27458–27465. https://doi.org/10.1074/jbc.M401009200
Sevilla A et al (2004) c-Jun phosphorylation by the human vaccinia-related kinase 1 (VRK1) and its cooperation with the N-terminal kinase of c-Jun (JNK). Oncogene 23(55):8950–8958. https://doi.org/10.1038/sj.onc.1208015
Lopez-Borges S, Lazo PA (2000) The human vaccinia-related kinase 1 (VRK1) phosphorylates threonine-18 within the mdm-2 binding site of the p53 tumour suppressor protein Oncogene, 19(32): p. 3656-64. https://doi.org/10.1038/sj.onc.1203709
López-Sánchez I et al (2014) VRK1 interacts with p53 forming a basal complex that is activated by UV-induced DNA damage. FEBS Lett 588(5):692–700. https://doi.org/10.1016/j.febslet.2014.01.040
Jiang J et al (2020) Type I interferons in the Pathogenesis and treatment of Autoimmune diseases. Clin Rev Allergy Immunol 59(2):248–272. https://doi.org/10.1007/s12016-020-08798-2
Zhu Y et al (2019) STING: a master regulator in the cancer-immunity cycle. Mol Cancer 18(1):152. https://doi.org/10.1186/s12943-019-1087-y
Chasset F, Dayer JM, Chizzolini C (2021) Type I interferons in systemic autoimmune diseases: distinguishing between afferent and efferent functions for Precision Medicine and Individualized Treatment. Front Pharmacol 12:633821. https://doi.org/10.3389/fphar.2021.633821
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province (BK20221029).
Author information
Authors and Affiliations
Contributions
M.P. and H.C. wrote the main manuscript text and Z.F. and X.W. prepared Figs. 1, 2, 3, 4, 5 and 6. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, with approval of the Institutional Ethics Committee of China Pharmaceutical University (Approval Number 2022–04–002).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, Z., Wang, X., Cheng, H. et al. VRK1 promotes DNA-induced type I interferon production. Mol Biol Rep 51, 453 (2024). https://doi.org/10.1007/s11033-024-09414-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09414-8