Abstract
Background
Natural products have been recommended as a complementary therapy for type 2 diabetes mellitus (T2DM) due to constraints of safety and tolerability of existing anti-diabetic agents. Luteolin exhibits anti-diabetic and anti-inflammatory effects. Hence, the impact of luteolin on glucose homoeostasis and organ damage was investigated in high-fat diet (HFD) and streptozotocin (STZ) induced T2DM in rats.
Methods and results
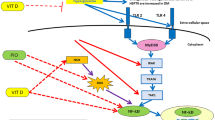
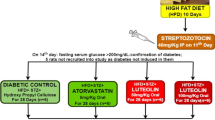
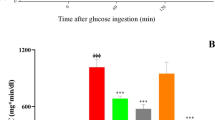
Male Wistar rats were maintained on HFD (provided 55% energy as fat) for 10 days. Subsequently, a single dose of 40 mg/kg STZ was injected intraperitoneally on the 11th day. Seventy-two hours after STZ administration, diabetic rats with established hyperglycemia (fasting serum glucose > 200 mg/dL) were randomized into different groups having six rats each and orally administered either 0.5% hydroxy propyl cellulose or pioglitazone (10 mg/kg) or luteolin (50 mg/kg or 100 mg/kg) once daily for 28 days, while continuing HFD for respective groups. Luteolin significantly reduced hyperglycaemia, homoeostasis model assessment (HOMA) of insulin resistance (HOMA-IR) levels, and improved hypoinsulinemia and HOMA of b-cell function (HOMA-B) in a dose-dependent manner. Increased TNF-α, IL-6 and NFκB levels in diabetic rats were significantly regulated. Additionally, luteolin significantly augmented PPAR-γ expression while attenuating sterol regulatory element binding protein-1c (SREBP-1c) expression. Histopathological scrutiny validated that luteolin effectively attenuated HFD-STZ-induced injury in pancreatic β-cells and kidneys to near normalcy.
Conclusion
Our study showed that luteolin ameliorated hyperglycemia and improved hypoinsulinemia, β-cell dysfunction, and renal impairment in HFD-STZ-induced diabetic rats by attenuating inflammation and dysregulated cytokine secretion through modulation of PPAR-γ, TNF-α, IL-6 and NF-kB expression and down-regulation of SREBP-1c.





Similar content being viewed by others
Data availability
The data generated during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- T2DM:
-
Type 2 diabetes mellitus
- HFD:
-
High-fat diet
- STZ:
-
Streptozotocin
- HOMA:
-
Homoeostasis model assessment
- HOMA-IR:
-
Homoeostasis model assessment of insulin resistance
- HOMA-B:
-
Homoeostasis model assessment of β-cell function
- IR:
-
Insulin resistance
- TNF-α:
-
Tumor necrosis factor-α
- IL-6:
-
Interleukin-6
- NFκB:
-
Nuclear factor-κB
- PPAR-γ:
-
Peroxisome proliferator-activated receptor-γ
- SREBP-1c:
-
Sterol regulatory element binding protein-1c
- HPC:
-
Hydroxy propyl cellulose
- QUICKI:
-
Quantitative Insulin Sensitivity Check Index
References
Saeedi P, Petersohn I, Salpea P et al (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 157:107843. https://doi.org/10.1016/j.diabres.2019.107843
Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL (2017) Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis 11:215–225. https://doi.org/10.1177/1753944717711379
Wellen KE (2005) Inflammation, stress, and diabetes. J Clin Investig 115:1111–1119. https://doi.org/10.1172/JCI200525102
Ricote M, Glass CK (2007) PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta 1771:926–935. https://doi.org/10.1016/j.bbalip.2007.02.013
Ferré P, Foufelle F (2007) SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res 68:72–82. https://doi.org/10.1159/000100426
Luo Y, Shang P, Li D (2017) Luteolin: a flavonoid that has multiple cardio-protective effects and its molecular mechanisms. Front Pharmacol 8:1–10. https://doi.org/10.3389/fphar.2017.00692
Aziz N, Kim MY, Cho JY (2018) Anti-inflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J Ethnopharmacol 225:342–358. https://doi.org/10.1016/j.jep.2018.05.019
El-Bassossy HM, Abo-Warda SM, Fahmy A (2014) Chrysin and luteolin alleviate vascular complications associated with insulin resistance mainly through PPAR-γ activation. Am J Chin Med 42:1153–1167. https://doi.org/10.1142/S0192415X14500724
Li L, Luo W, Qian Y et al (2019) Luteolin protects against diabetic cardiomyopathy by inhibiting NF-κB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine. https://doi.org/10.1016/j.phymed.2018.11.034
Wang GG, Lu XH, Li W et al (2011) Protective effects of luteolin on diabetic nephropathy in STZ-induced diabetic rats. Evid Based Complement Altern Med. https://doi.org/10.1155/2011/323171
Lu HE, Chen Y, Sun XB et al (2015) Effects of luteolin on retinal oxidative stress and inflammation in diabetes. RSC Adv 5:4898–4904. https://doi.org/10.1039/c4ra10756j
Gheibi S, Kashfi K, Ghasemi A (2017) A practical guide for induction of type-2 diabetes in rat: incorporating a high-fat diet and streptozotocin. Biomed Pharmacother 95:605–613. https://doi.org/10.1016/J.BIOPHA.2017.08.098
Srinivasan K, Viswanad B, Asrat L et al (2005) Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res 52:313–320. https://doi.org/10.1016/j.phrs.2005.05.004
Vital P, Larrieta E, Hiriart M (2006) Sexual dimorphism in insulin sensitivity and susceptibility to develop diabetes in rats. J Endocrinol 190:425–432. https://doi.org/10.1677/joe.1.06596
Shehnaz SI, Roy A, Vijayaraghavan R, Sivanesan S (2023) Luteolin mitigates diabetic dyslipidemia in rats by modulating ACAT-2, PPARα, SREBP-2 proteins, and oxidative stress. Appl Biochem Biotechnol 195:4893–4914. https://doi.org/10.1007/s12010-023-04544-4
Albasher G (2020) Modulation of reproductive dysfunctions associated with streptozocin-induced diabetes by Artemisia judaica extract in rats fed a high-fat diet. Mol Biol Rep 47:7517–7527. https://doi.org/10.1007/s11033-020-05814-8
Sharma AK, Bharti S, Ojha S et al (2011) Up-regulation of PPARγ, heat shock protein-27 and -72 by naringin attenuates insulin resistance, β-cell dysfunction, hepatic steatosis and kidney damage in a rat model of type 2 diabetes. Br J Nutr 106:1713–1723. https://doi.org/10.1017/S000711451100225X
Abu-Elsaad N, El-Karef A (2018) The falconoid luteolin mitigates the myocardial inflammatory response induced by high-carbohydrate/high-fat diet in wistar rats. Inflammation 41:221–231. https://doi.org/10.1007/s10753-017-0680-8
Matthews DR, Hosker JP, Rudenski AS et al (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419. https://doi.org/10.1007/BF00280883
Katz A, Nambi SS, Mather K et al (2000) Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85:2402–2410. https://doi.org/10.1210/jcem.85.7.6661
Bancroft J, Gamble M (2008) Theory and practice of histopathological techniques, 6th edn. Churchill Livingstone, Philadelphia
Tayeb W, Nakbi A, Trabelsi M et al (2012) Biochemical and histological evaluation of kidney damage after sub-acute exposure to 2,4-dichlorophenoxyacetic herbicide in rats: involvement of oxidative stress. Toxicol Mech Methods 22:696–704. https://doi.org/10.3109/15376516.2012.717650
Elaidy SM, Hussain MA, El-Kherbetawy MK (2018) Time-dependent therapeutic roles of nitazoxanide on high-fat diet/streptozotocin-induced diabetes in rats: effects on hepatic peroxisome proliferator-activated receptor-gamma receptors. Can J Physiol Pharmacol 96:485–497. https://doi.org/10.1139/cjpp-2017-0533
Soetikno V, Andini P, Iskandar M et al (2023) Alpha-mangosteen lessens high-fat/high-glucose diet and low-dose streptozotocin induced-hepatic manifestations in the insulin resistance rat model. Pharm Biol 61:241–248. https://doi.org/10.1080/13880209.2023.2166086
Zang Y, Igarashi K, Li YL (2016) Anti-diabetic effects of luteolin and luteolin-7-O-glucoside on KK-Ay mice. Biosci Biotechnol Biochem 80:1580–1586. https://doi.org/10.1080/09168451.2015.1116928
Liu Y, Fu X, Lan N et al (2014) Luteolin protects against high fat diet-induced cognitive deficits in obesity mice. Behav Brain Res 267:178–188. https://doi.org/10.1016/j.bbr.2014.02.040
Xu N, Zhang L, Dong J et al (2014) Low-dose diet supplement of a natural flavonoid, luteolin, ameliorates diet-induced obesity and insulin resistance in mice. Mol Nutr Food Res 58:1258–1268. https://doi.org/10.1002/mnfr.201300830
Kwon EY, Jung UJ, Park T et al (2015) Luteolin attenuates hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue in mice with diet-induced obesity. Diabetes 64:1658–1669. https://doi.org/10.2337/db14-0631
Ding L, Jin D, Chen X (2010) Luteolin enhances insulin sensitivity via activation of PPARγ transcriptional activity in adipocytes. J Nutr Biochem 21:941–947. https://doi.org/10.1016/j.jnutbio.2009.07.009
Deqiu Z, Kang L, Jiali Y et al (2011) Luteolin inhibits inflammatory response and improves insulin sensitivity in the endothelium. Biochimie 93:506–512. https://doi.org/10.1016/j.biochi.2010.11.002
Guo F, Xu S, Zhu Y et al (2020) PPARγ transcription deficiency exacerbates high-fat diet-induced adipocyte hypertrophy and insulin resistance in mice. Front Pharmacol 11:1285. https://doi.org/10.3389/fphar.2020.01285
Li B, Du P, Du Y et al (2021) Luteolin alleviates inflammation and modulates gut microbiota in ulcerative colitis rats. Life Sci 269:119008. https://doi.org/10.1016/j.lfs.2020.119008
Puhl AC, Bernardes A, Silveira RL et al (2012) Mode of peroxisome proliferator-activated receptor γ activation by luteolin. Mol Pharmacol 81:788–799. https://doi.org/10.1124/mol.111.076216
Chen L, Tian G, Tang W et al (2016) Protective effect of luteolin on streptozotocin-induced diabetic renal damage in mice via the regulation of RIP140/NF-ΚB pathway and insulin signalling pathway. J Funct Foods 22:93–100. https://doi.org/10.1016/j.jff.2016.01.023
Sun D, Huang J, Zhang Z et al (2012) Luteolin limits infarct size and improves cardiac function after myocardium ischemia/reperfusion injury in diabetic rats. PLoS ONE 7:1–10. https://doi.org/10.1371/journal.pone.0033491
Horton JD, Bashmakov Y, Shimomura I, Shimano H (1998) Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci USA 95:5987–5992. https://doi.org/10.1073/pnas.95.11.5987
Liu JF, Ma Y, Wang Y et al (2011) Reduction of lipid accumulation in HepG2 cells by luteolin is associated with activation of AMPK and mitigation of oxidative stress. Phyther Res 25:588–596. https://doi.org/10.1002/ptr.3305
Yin Y, Gao L, Lin H et al (2017) Luteolin improves non-alcoholic fatty liver disease in db/db mice by inhibition of liver X receptor activation to down-regulate expression of sterol regulatory element binding protein 1c. Biochem Biophys Res Commun 482:720–726. https://doi.org/10.1016/j.bbrc.2016.11.101
Lee FTH, Cao Z, Long DM et al (2004) Interactions between angiotensin II and NF-κB-dependent pathways in modulating macrophage infiltration in experimental diabetic nephropathy. J Am Soc Nephrol 15:2139–2151. https://doi.org/10.1097/01.ASN.0000135055.61833.A8
Zhang M, He L, Liu J, Zhou L (2021) Luteolin attenuates diabetic nephropathy through suppressing inflammatory response and oxidative stress by inhibiting STAT3 pathway. Exp Clin Endocrinol Diabetes 129:729–739. https://doi.org/10.1055/a-0998-7985
Albarakati AJA, Baty RS, Aljoudi AM et al (2020) Luteolin protects against lead acetate-induced nephrotoxicity through antioxidant, anti-inflammatory, anti-apoptotic, and Nrf2/HO-1 signaling pathways. Mol Biol Rep 47:2591–2603. https://doi.org/10.1007/s11033-020-05346-1
Kalbolandi SM, Gorji AV, Babaahmadi-Rezaei H, Mansouri E (2019) Luteolin confers renoprotection against ischemia–reperfusion injury via involving Nrf2 pathway and regulating miR320. Mol Biol Rep 46:4039–4047. https://doi.org/10.1007/s11033-019-04853-0
Fu Z, Gilbert ER, Liu D (2013) Regulation of insulin synthesis and secretion and pancreatic beta-cell dysfunction in diabetes. Curr Diabetes Rev 9:25–53
Kim EK, Kwon KB, Song MY et al (2007) Flavonoids protect against cytokine-induced pancreatic β-cell damage through suppression of nuclear factor κb activation. Pancreas 35:1–9. https://doi.org/10.1097/mpa.0b013e31811ed0d2
Ding Y, Shi X, Shuai X et al (2014) Luteolin prevents uric acid-induced pancreatic β-cell dysfunction. J Biomed Res 28:292–298. https://doi.org/10.7555/JBR.28.20130170
Orji CE, Okpoko CK, Agbata CA et al (2020) Evaluation of the effect of luteolin on the hepatic and hematopoietic systems in albino rats. J Clin Toxicol 10:2–6. https://doi.org/10.35248/2161-0495.20.10.434
Xiong J, Wang K, Yuan C et al (2017) Luteolin protects mice from severe acute pancreatitis by exerting HO-1-mediated anti-inflammatory and antioxidant effects. Int J Mol Med 39:113–125. https://doi.org/10.3892/ijmm.2016.2809
Lin LC, Pai YF, Tsai TH (2015) Isolation of luteolin and luteolin-7-O-glucoside from Dendranthema morifolium Ramat Tzvel and their pharmacokinetics in rats. J Agric Food Chem 63:7700–7706. https://doi.org/10.1021/jf505848z
Dang H, Meng MHW, Zhao H et al (2014) Luteolin-loaded solid lipid nanoparticles synthesis, characterization, & improvement of bioavailability, pharmacokinetics in vitro and vivo studies. J Nanopart Res. https://doi.org/10.1007/s11051-014-2347-9
Sinha A, Suresh PK (2019) Enhanced induction of apoptosis in HaCaT cells by luteolin encapsulated in PEGylated liposomes—role of caspase-3/caspase-14. Appl Biochem Biotechnol 188:147–164. https://doi.org/10.1007/s12010-018-2907-z
Acknowledgements
The authors acknowledge the technical support rendered by Mr. P. Praveen Kumar, Laboratory assistant of the Department of Research and Development, Saveetha Institute of Medical and Technical Sciences, Chennai, India during the study.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
SIS conceived and designed the experiments, guided by AR. SIS performed the experiments, guided by RV and SS. NP and SIS contributed to the analysis and interpretation of histopathological data. SIS and RV performed the statistical analysis. The first draft of the manuscript was written by SIS with contributions to the methodology section by RV, SS and NP. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. The authors declare that all data was generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The experiments were designed in accordance with the Committee for Control and Supervision of Experiments on Animals; Ministry of Forests, Environment and Climate Change, Government of India, and were approved by the Institutional Animal Ethics Committee (Approval No. SU/CLAR/RD/007/ 2019, dated: 21.12.2019).
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shehnaz, S.I., Roy, A., Vijayaraghavan, R. et al. Modulation of PPAR-γ, SREBP-1c and inflammatory mediators by luteolin ameliorates β-cell dysfunction and renal damage in a rat model of type-2 diabetes mellitus. Mol Biol Rep 50, 9129–9142 (2023). https://doi.org/10.1007/s11033-023-08804-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08804-8