Abstract
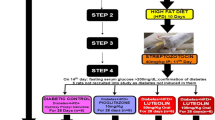
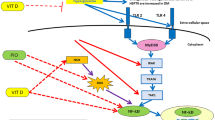
Diabetic dyslipidemia is a crucial link between type-2 diabetes mellitus (T2DM) and atherosclerotic cardiovascular diseases (ASCVD). Natural biologically active substances have been advocated as complementary remedies for ASCVD and T2DM. Luteolin, a flavonoid, exhibits antioxidant, hypolipidemic, and antiatherogenic effects. Hence, we aimed to determine influence of luteolin on lipid homeostasis and hepatic damage in rats with T2DM induced by high-fat-diet (HFD) and streptozotocin (STZ). After being fed HFD for 10 days, male Wistar rats received 40 mg/kg STZ intraperitoneal injection on 11th day. Seventy-two hours later, hyperglycemic rats (fasting glucose > 200 mg/dL) were randomized into groups, and oral hydroxy-propyl-cellulose, atorvastatin (5 mg/kg), or luteolin (50 mg/kg or 100 mg/kg) administered daily, while continuing HFD for 28 days. Luteolin significantly ameliorated dyslipidemia levels and concomitantly improved atherogenic index of plasma in a dose-dependent manner. Increased levels of malondialdehyde and diminished levels of superoxide dismutase, catalase, and glutathione in HFD-STZ-diabetic rats were significantly regulated by luteolin. Luteolin significantly intensified PPARα expression while decreasing expression of acyl-coenzyme A:cholesterol acyltransferase-2 (ACAT-2) and sterol regulatory element binding protein-2 (SREBP-2) proteins. Moreover, luteolin effectively alleviated hepatic impairment in HFD-STZ-diabetic rats to near-normal control levels. The findings of the present study expound mechanisms by which luteolin mitigated diabetic dyslipidemia and alleviated hepatic impairment in HFD-STZ-diabetic rats by amelioration of oxidative stress, modulation of PPARα expression, and downregulation of ACAT-2 and SREBP-2. In conclusion, our results imply that luteolin may be efficacious in management of dyslipidemia in T2DM, and future research may be essential to substantiate our findings.






Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
World Health Organization (WHO). (2021). Cardiovascular diseases: Fact sheet No. 317 from http://www.who.int/mediacentre/factsheets/fs317/en/ . Accessed 30 Oct 2022.
Saeedi, P., Petersohn, I., Salpea, P., Malanda, B., Karuranga, S., Unwin, N., & Williams, R. (2019). Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Research and Clinical Practice, 157, 107843. https://doi.org/10.1016/j.diabres.2019.107843
McFarlane, S. I., Banerji, M., & Sowers, J. R. (2001). Insulin resistance and cardiovascular disease. The Journal of Clinical Endocrinology & Metabolism, 86(2), 713–718. https://doi.org/10.1210/JCEM.86.2.7202
Tangvarasittichai, S. (2015). Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World Journal of Diabetes, 6(3), 456. https://doi.org/10.4239/wjd.v6.i3.456
Del Rio, D., Stewart, A. J., & Pellegrini, N. (2005). A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutrition, Metabolism and Cardiovascular Diseases, 15(4), 316–328. https://doi.org/10.1016/j.numecd.2005.05.003
Libby, P., Ridker, P. M., & Hansson, G. K. (2011). Progress and challenges in translating the biology of atherosclerosis. Nature, 473(7347), 317–325. https://doi.org/10.1038/nature10146
Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., Squadrito, F., Altavilla, D., & Bitto, A. (2017). Oxidative stress harms and benefits for human health. Oxidative Medicine and Cellular Longevity, 2017, 8416763. https://doi.org/10.1155/2017/8416763
Yu, X. H., Zheng, X. L., & Tang, C. K. (2015). Peroxisome proliferator-activated receptor α in lipid metabolism and atherosclerosis in advances in clinical chemistry (1st ed., Vol. 71, pp. 171–203). Elsevier Inc. https://doi.org/10.1016/bs.acc.2015.06.005
Eberlé, D., Hegarty, B., Bossard, P., Ferré, P., & Foufelle, F. (2004). SREBP transcription factors: Master regulators of lipid homeostasis. Biochimie, 86(11), 839–848. https://doi.org/10.1016/j.biochi.2004.09.018
Hai, Q., & Smith, J. D. (2021). Acyl-coenzyme a: Cholesterol acyltransferase (acat) in cholesterol metabolism: From its discovery to clinical trials and the genomics era. Metabolites, 11(8), 543. https://doi.org/10.3390/metabo11080543
Hori, M., Satoh, M., Furukawa, K., Sakamoto, Y. I., Hakamata, H., Komohara, Y., & Horiuchi, S. (2004). Acyl-coenzyme A:cholesterol acyltransferase-2 (ACAT-2) is responsible for elevated intestinal ACAT activity in diabetic rats. Arteriosclerosis, Thrombosis, and Vascular Biology, 24(9), 1689–1695. https://doi.org/10.1161/01.ATV.0000137976.88533.13
Newman, D., & Cragg, G. (2020). Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. Journal of Natural Products, 83(3), 770–803. https://doi.org/10.1021/acs.jnatprod.9b01285
Wang, Z., Zeng, M., Wang, Z., Qin, F., Chen, J., & He, Z. (2021). Dietary luteolin: A narrative review focusing on its pharmacokinetic properties and effects on glycolipid metabolism. Journal of Agricultural and Food Chemistry, 69(5), 1441–1454. https://doi.org/10.1021/acs.jafc.0c08085
Luo, Y., Shang, P., & Li, D. (2017). Luteolin: A flavonoid that has multiple cardio-protective effects and its molecular mechanisms. Frontiers in Pharmacology, 8(OCT), 1–10. https://doi.org/10.3389/fphar.2017.00692
Seelinger, G., Merfort, I., & Schempp, C. M. (2008). Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Medica, 74(14), 1667–1677. https://doi.org/10.1055/s-0028-1088314
Wang, G., Li, W., Lu, X., Bao, P., & Zhao, X. (2012). Luteolin ameliorates cardiac failure in type I diabetic cardiomyopathy. Journal of Diabetes and its Complications, 26(4), 259–265. https://doi.org/10.1016/j.jdiacomp.2012.04.007
Wang, G. G., Lu, X. H., Li, W., Zhao, X., & Zhang, C. (2011). Protective effects of luteolin on diabetic nephropathy in STZ-induced diabetic rats. Evidence based Complementary and Alternative Medicine, 2011, 323171. https://doi.org/10.1155/2011/323171
Li, L., Luo, W., Qian, Y., Zhu, W., Qian, J., Li, J., ... & Liang, G. (2019). Luteolin protects against diabetic cardiomyopathy by inhibiting NF-κB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine, 59, 152774. https://doi.org/10.1016/j.phymed.2018.11.034
Abu-Elsaad, N., & El-Karef, A. (2018). The falconoid luteolin mitigates the myocardial inflammatory response induced by high-carbohydrate/high-fat diet in Wistar rats. Inflammation, 41(1), 221–231. https://doi.org/10.1007/s10753-017-0680-8
Skovsø, S. (2014). Modeling type 2 diabetes in rats using high fat diet and streptozotocin. Journal of Diabetes Investigation, 5(4), 349–358. https://doi.org/10.1111/jdi.12235
Srinivasan, K., Viswanad, B., Asrat, L., Kaul, C. L., & Ramarao, P. (2005). Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacological Research, 52(4), 313–320. https://doi.org/10.1016/j.phrs.2005.05.004
Vital, P., Larrieta, E., & Hiriart, M. (2006). Sexual dimorphism in insulin sensitivity and susceptibility to develop diabetes in rats. Journal of Endocrinology, 190(2), 425–432. https://doi.org/10.1677/joe.1.06596
Gheibi, S., Kashfi, K., & Ghasemi, A. (2017). A practical guide for induction of type-2 diabetes in rat: Incorporating a high-fat diet and streptozotocin. Biomedicine & Pharmacotherapy, 95, 605–613. https://doi.org/10.1016/J.BIOPHA.2017.08.098
Sharma, A. K., Bharti, S., Ojha, S., Bhatia, J., Kumar, N., Ray, R., & Arya, D. S. (2011). Up-regulation of PPARγ, heat shock protein-27 and-72 by naringin attenuates insulin resistance, β-cell dysfunction, hepatic steatosis and kidney damage in a rat model of type 2 diabetes. British Journal of Nutrition, 106(11), 1713–1723. https://doi.org/10.1017/S000711451100225X
Shyamala, M. P., Venukumar, M. R., & Latha, M. S. (2003). Antioxidant potential of the Syzygium aromaticum (Gaertn.) Linn. (cloves) in rats fed with high fat diet. Indian Journal of Pharmacology, 35(2), 99–103.
Banerjee, A., Das, D., Paul, R., Roy, S., Bhattacharjee, A., Prasad, S. K., & Maji, B. K. (2020). Altered composition of high-lipid diet may generate reactive oxygen species by disturbing the balance of antioxidant and free radicals. Journal of Basic and Clinical Physiology and Pharmacology, 31(3), 1–19. https://doi.org/10.1515/jbcpp-2019-0141
Zhang, W. L., Yan, W. J., Sun, B., & Zou, Z. P. (2014). Synergistic effects of atorvastatin and rosiglitazone on endothelium protection in rats with dyslipidemia. Lipids in Health and Disease, 13(1), 1–5. https://doi.org/10.1186/1476-511X-13-168
Liu, Y., Tian, X., Gou, L., Sun, L., Ling, X., & Yin, X. (2013). Luteolin attenuates diabetes-associated cognitive decline in rats. Brain Research Bulletin, 94, 23–29. https://doi.org/10.1016/j.brainresbull.2013.02.001
Yang, J. T., Wang, J., Zhou, X. R., Xiao, C., Lou, Y. Y., Tang, L. H., & Qian, L. B. (2018). Luteolin alleviates cardiac ischemia/reperfusion injury in the hypercholesterolemic rat via activating Akt/Nrf2 signaling. Naunyn-Schmiedeberg’s Archives of Pharmacology, 391(7), 719–728. https://doi.org/10.1007/s00210-018-1496-2
Friedewald, W., Levy, R., & Fredrickson, D. (1972). Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry, 18(6), 499–502.
Dobiášová, M., & Frohlich, J. (2001). The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate inapob-lipoprotein-depleted plasma (FERHDL). Clinical Biochemistry, 34(7), 583–588. https://doi.org/10.1016/S0009-9120(01)00263-6
Mozaffari Godarzi, S., Valizade Gorji, A., Gholizadeh, B., Mard, S. A., & Mansouri, E. (2020). Antioxidant effect of p-coumaric acid on interleukin 1-β and tumor necrosis factor-α in rats with renal ischemic reperfusion. Nefrología, 40(3), 311–319. https://doi.org/10.1016/j.nefroe.2020.06.017
Boriskin, P., Deviatkin, A., Nikitin, A., Pavlova, O., & Toropovskiy, A. (2019). Relationship of catalase activity distribution in serum and tissues of small experimental animals. IOP Conference Series Earth and Environmental Science, 403(1), 012113. https://doi.org/10.1088/1755-1315/403/1/012113
Ellman, G. L. (1959). Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics, 82(1), 70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Elkadri, A., Thoeni, C., Deharvengt, S. J., Murchie, R., Guo, C., Stavropoulos, J. D., Marshall, C. R., Wales, P., Bandsma, R., Cutz, E., Roifman, C. M., Chitayat, D., Avitzur, Y., Stan, R. V., & Muise, A. M. (2015). Mutations in plasmalemma vesicle associated protein result in sieving protein-losing enteropathy characterized by hypoproteinemia, hypoalbuminemia, and hypertriglyceridemia. Cell Mol Gastroenterol Hepatol, 1(4), 381–394. https://doi.org/10.1016/j.jcmgh.2015.05.001
Xu, G., Müller, O., Stange, E. F., & Fuchs, M. (2004). Impaired regulation of sterol regulatory element binding protein 2 in cholesterol gallstone-susceptible mice. Biochimica et Biophysica Acta - Molecular Basis of Disease, 1688(3), 274–279. https://doi.org/10.1016/j.bbadis.2004.01.001
Bancroft, J., & Gamble, M. (2008). Theory and practice of histopathological techniques (6th ed.). Churchill Livingstone.
Jang, A., Srinivasan, P., Lee, N. Y., Song, H. P., Lee, J. W., Lee, M., & Jo, C. (2008). Comparison of hypolipidemic activity of synthetic gallic acid-linoleic acid ester with mixture of gallic acid and linoleic acid, gallic acid, and linoleic acid on high-fat diet induced obesity in C57BL/6 Cr Slc mice. Chemico-Biological Interactions, 174(2), 109–117. https://doi.org/10.1016/j.cbi.2008.05.018
Sharma, A. K., Bharti, S., Goyal, S., Arora, S., Nepal, S., Kishore, K., & Arya, D. S. (2011). Upregulation of PPARγ by Aegle marmelos ameliorates insulin resistance and β-cell dysfunction in high fat diet fed-streptozotocin induced type 2 diabetic rats. Phytotherapy Research, 25(10), 1457–1465. https://doi.org/10.1002/ptr.3442
C. Thambiah, S., & Lai, L. C. (2021). Diabetic dyslipidaemia. Practical Laboratory Medicine, 26(May), e00248. https://doi.org/10.1016/j.plabm.2021.e00248
Zang, Y., Igarashi, K., & Li, Y. L. (2016). Anti-diabetic effects of luteolin and luteolin-7-O-glucoside on KK-Ay mice. Bioscience, Biotechnology and Biochemistry, 80(8), 1580–1586. https://doi.org/10.1080/09168451.2015.1116928
Chen, L., Tian, G., Tang, W., Luo, W., Liu, P., & Ma, Z. (2016). Protective effect of luteolin on streptozotocin-induced diabetic renal damage in mice via the regulation of RIP140/NF-ΚB pathway and insulin signalling pathway. Journal of Functional Foods, 22, 93–100. https://doi.org/10.1016/j.jff.2016.01.023
Wong, T. Y., Tan, Y. Q., Lin, S. M., & Leung, L. K. (2017). Apigenin and luteolin display differential hypocholesterolemic mechanisms in mice fed a high-fat diet. Biomedicine and Pharmacotherapy, 96(November), 1000–1007. https://doi.org/10.1016/j.biopha.2017.11.131
Zhu, Y., Liu, R., Shen, Z., & Cai, G. (2020). Combination of luteolin and lycopene effectively protect against the “two-hit” in NAFLD through Sirt1/AMPK signal pathway. Life Sciences, 256(June), 117990. https://doi.org/10.1016/j.lfs.2020.117990
Li, J., Inoue, J., Choi, J. M., Nakamura, S., Yan, Z., Fushinobu, S., & Sato, R. (2015). Identification of the flavonoid luteolin as a repressor of the transcription factor hepatocyte nuclear factor 4α. Journal of Biological Chemistry, 290(39), 24021–24035. https://doi.org/10.1074/jbc.M115.645200
Gentile, D., Fornai, M., Pellegrini, C., Colucci, R., Benvenuti, L., Duranti, E., Antonioli, L. (2018). Luteolin prevents cardiometabolic alterations and vascular dysfunction in mice with HFD-induced obesity. Frontiers in Pharmacology, 9(SEP);1–13. https://doi.org/10.3389/fphar.2018.01094
Park, H. S., Lee, K., Kim, S. H., Hong, M. J., Jeong, N. J., & Kim, M. S. (2020). Luteolin improves hypercholesterolemia and glucose intolerance through LXRα-dependent pathway in diet-induced obese mice. Journal of Food Biochemistry, 44(9), 1–9. https://doi.org/10.1111/jfbc.13358
Kwon, E. Y., Jung, U. J., Park, T., Yun, J. W., & Choi, M. S. (2015). Luteolin attenuates hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue in mice with diet-induced obesity. Diabetes, 64(5), 1658–1669. https://doi.org/10.2337/db14-0631
Liu, G., Zhang, Y., Liu, C., Xu, D., Zhang, R., Cheng, Y., & Chen, Y. (2014). Luteolin alleviates alcoholic liver disease induced by chronic and binge ethanol feeding in mice. Journal of Nutrition, 144(7), 1009–1015. https://doi.org/10.3945/jn.114.193128
El-Bassossy, H. M., Abo-Warda, S. M., & Fahmy, A. (2014). Chrysin and luteolin alleviate vascular complications associated with insulin resistance mainly through PPAR-γ activation. American Journal of Chinese Medicine, 42(5), 1153–1167. https://doi.org/10.1142/S0192415X14500724
Zhang, Y., Tian, X. Q., Song, X. X., Ge, J. P., & Xu, Y. C. (2017). Luteolin protect against diabetic cardiomyopathy in rat model via regulating the AKT/GSK-3β signalling pathway. Biomedical Research (India), 28(3), 1359–1363.
El-Bassossy, H. M., Abo-Warda, S. M., & Fahmy, A. (2013). Chrysin and luteolin attenuate diabetes-induced impairment in endothelial-dependent relaxation: Effect on lipid profile, AGEs and NO generation. Phytotherapy Research, 27(11), 1678–1684. https://doi.org/10.1002/ptr.4917
Nekohashi, M., Ogawa, M., Ogihara, T., Nakazawa, K., Kato, H., Misaka, T., & Kobayashi, S. (2014). Luteolin and quercetin affect the cholesterol absorption mediated by epithelial cholesterol transporter Niemann-Pick C1-Like 1 in Caco-2 cells and rats. PLoS ONE, 9(5), 1–9. https://doi.org/10.1371/journal.pone.0097901
Yin, Y., Gao, L., Lin, H., Wu, Y., Han, X., Zhu, Y., & Li, J. (2017). Luteolin improves non-alcoholic fatty liver disease in db/db mice by inhibition of liver X receptor activation to down-regulate expression of sterol regulatory element binding protein 1c. Biochemical and Biophysical Research Communications, 482(4), 720–726. https://doi.org/10.1016/j.bbrc.2016.11.101
Shon, J. C., Kim, W. C., Ryu, R., Wu, Z., Seo, J. S., Choi, M. S., & Liu, K. H. (2020). Plasma lipidomics reveals insights into anti-obesity effect of chrysanthemum morifolium ramat leaves and its constituent luteolin in high-fat diet-induced dyslipidemic mice. Nutrients, 12(10), 1–15. https://doi.org/10.3390/nu12102973
Kwon, E. Y., Kim, S. Y., & Choi, M. S. (2018). Luteolin-enriched artichoke leaf extract alleviates the metabolic syndrome in mice with high-fat diet-induced obesity. Nutrients, 10(8), 979. https://doi.org/10.3390/nu10080979
Lu, H. E., Chen, Y., Sun, X. B., Tong, B., & Fan, X. H. (2015). Effects of luteolin on retinal oxidative stress and inflammation in diabetes. RSC Advances, 5(7), 4898–4904. https://doi.org/10.1039/c4ra10756j
Guo, X., Wang, X., Wang, Y., Ji, K., Ji, B. P., & Zhou, F. (2018). Stability of a type 2 diabetes rat model induced by high-fat diet feeding with low-dose streptozotocin injection. Journal of Zhejiang University: Science B, 19(7), 559–569. https://doi.org/10.1631/jzus.B1700254
Giacco, F., & Brownlee, M. (2010). Oxidative stress and diabetic complications. Circulation Research, 107(9), 1058–1070. https://doi.org/10.1161/CIRCRESAHA.110.223545/FORMAT/EPUB
Zhang, T., Wu, W., Li, D., Xu, T., Zhu, H., Pan, D., & Liu, Y. (2014). Anti-oxidant and anti-apoptotic effects of luteolin on mice peritoneal macrophages stimulated by angiotensin II. International Immunopharmacology, 20(2), 346–351. https://doi.org/10.1016/j.intimp.2014.03.018
Kwon, E. Y., & Choi, M. S. (2018). Luteolin targets the toll-like receptor signaling pathway in prevention of hepatic and adipocyte fibrosis and insulin resistance in diet-induced obese mice. Nutrients, 10(10), 1–17. https://doi.org/10.3390/nu10101415
Abu-Elsaad, N., & El-Karef, A. (2019). Protection against nonalcoholic steatohepatitis through targeting IL-18 and IL-1alpha by luteolin. Pharmacological Reports, 71(4), 688–694. https://doi.org/10.1016/j.pharep.2019.03.009
Tai, M., Zhang, J., Song, S., Miao, R., Liu, S., Pang, Q., & Liu, C. (2015). Protective effects of luteolin against acetaminophen-induced acute liver failure in mouse. International Immunopharmacology, 27(1), 164–170. https://doi.org/10.1016/j.intimp.2015.05.009
Park, C. M., & Song, Y. S. (2019). Luteolin and luteolin-7-o-glucoside protect against acute liver injury through regulation of inflammatory mediators and antioxidative enzymes in GaLN/LPS-induced hepatitic ICR mice. Nutrition Research and Practice, 13(6), 473–479. https://doi.org/10.4162/nrp.2019.13.6.473
Zhang, H., Tan, X., Yang, D., Lu, J., Liu, B., Baiyun, R., & Zhang, Z. (2017). Dietary luteolin attenuates chronic liver injury induced by mercuric chloride via the Nrf2/NF-κB/P53 signaling pathway in rats. Oncotarget, 8(25), 40982–40993. https://doi.org/10.18632/oncotarget.17334
Yang, D., Tan, X., Lv, Z., Liu, B., Baiyun, R., Lu, J., & Zhang, Z. (2016). Regulation of Sirt1/Nrf2/TNF-α signaling pathway by luteolin is critical to attenuate acute mercuric chloride exposure induced hepatotoxicity. Scientific Reports, 6(May), 1–12. https://doi.org/10.1038/srep37157
Samy, R. P., Gopalakrishnakone, P., & Ignacimuthu, S. (2006). Anti-tumor promoting potential of luteolin against 7,12-dimethylbenz(a)anthracene-induced mammary tumors in rats. Chemico-Biological Interactions, 164(1–2), 1–14. https://doi.org/10.1016/j.cbi.2006.08.018
Xu, H., Linn, B., Zhang, Y., & Ren, J. (2019). A review on the antioxidative and prooxidative properties of luteolin. Reactive Oxygen Species, 7(21), 136–147. https://doi.org/10.20455/ros.2019.833
Rogers, M. A., Liu, J., Song, B. L., Li, B. L., Chang, C. C. Y., & Chang, T. Y. (2015). Acyl-CoA:Cholesterol acyltransferases (ACATs/SOATs): Enzymes with multiple sterols as substrates and as activators. Journal of Steroid Biochemistry and Molecular Biology, 151, 102–107. https://doi.org/10.1016/j.jsbmb.2014.09.008
Kusunoki, J., Aragane, K., Kitamine, T., Kozono, H., Kano, K., Fujinami, K., & Sekine, Y. (2000). Postprandial hyperlipidemia in streptozotocin-induced diabetic rats is due to abnormal increase in intestinal acyl coenzyme A:cholesterol acyltransferase activity. Arteriosclerosis, Thrombosis, and Vascular Biology, 20(1), 171–178. https://doi.org/10.1161/01.ATV.20.1.171
Wang, J., Gao, T., Wang, F., Xue, J., Ye, H., & Xie, M. (2019). Luteolin improves myocardial cell glucolipid metabolism by inhibiting hypoxia inducible factor-1α expression in angiotensin II/hypoxia-induced hypertrophic H9c2 cells. Nutrition Research, 65, 63–70. https://doi.org/10.1016/j.nutres.2019.02.004
Horton, J. D., Goldstein, J. L., & Brown, M. S. (2002). SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. Journal of Clinical Investigation, 109(9), 1125–1131. https://doi.org/10.1172/JCI0215593
Edwards, P. A., Tabor, D., Kast, H. R., & Venkateswaran, A. (2000). Regulation of gene expression by SREBP and SCAP. Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids, 1529(1–3), 103–113. https://doi.org/10.1016/S1388-1981(00)00140-2
Ogawa, M., Yamanashi, Y., Takada, T., Abe, K., & Kobayashi, S. (2017). Effect of luteolin on the expression of intestinal cholesterol transporters. Journal of Functional Foods, 36, 274–279. https://doi.org/10.1016/J.JFF.2017.07.008
Wong, T. Y., Lin, S. M., & Leung, L. K. (2015). The flavone luteolin suppresses SREBP-2 expression and post-translational activation in hepatic cells. PLoS ONE, 10(8), 1–18. https://doi.org/10.1371/journal.pone.0135637
Tan, Y. Q., Wong, T. Y., Lin, S. M., & Leung, L. K. (2017). Dietary flavones counteract phorbol 12-myristate 13-acetate-induced SREBP-2 processing in hepatic cells. Molecular and Cellular Biochemistry, 424(1–2), 163–172. https://doi.org/10.1007/s11010-016-2851-6
Domitrović, R., Jakovac, H., Grebić, D., Milin, Č, & Radošević-Stašić, B. (2008). Dose- and time-dependent effects of luteolin on liver metallothioneins and metals in carbon tetrachloride-induced hepatotoxicity in mice. Biological Trace Element Research, 126(1–3), 176–185. https://doi.org/10.1007/s12011-008-8181-0
Orji, C. E., Okpoko, C. K., Agbata, C. A., Nnaemeka, J., Okeke, A. C., & Ihekwereme, C. P. (2020). Evaluation of the effect of luteolin on the hepatic and hematopoietic systems in albino rats. Journal of Clinical Toxicology, 10(1000434), 2–6.
Xiong, J., Wang, K., Yuan, C., Xing, R., Ni, J., Hu, G., & Wang, X. (2017). Luteolin protects mice from severe acute pancreatitis by exerting HO-1-mediated anti-inflammatory and antioxidant effects. International Journal of Molecular Medicine, 39(1), 113–125. https://doi.org/10.3892/ijmm.2016.2809
Czeczot, H., Tudek, B., Kusztelak, J., Szymczyk, T., Dobrowolska, B., Glinkowska, G., & Strzelecka, H. (1990). Isolation and studies of the mutagenic activity in the Ames test of flavonoids naturally occurring in medical herbs. Mutation Research/Genetic Toxicology, 240(3), 209–216. https://doi.org/10.1016/0165-1218(90)90060-F
Horváthová, K., Chalupa, I., Šebová, L., Tóthová, D., & Vachálková, A. (2005). Protective effect of quercetin and luteolin in human melanoma HMB-2 cells. Mutation Research - Genetic Toxicology and Environmental Mutagenesis, 565(2), 105–112. https://doi.org/10.1016/j.mrgentox.2004.08.013
Mittra, B., Saha, A., Chowdhury, A. R., Pal, C., Mandal, S., Mukhopadhyay, S., & Majumder, H. K. (2000). Luteolin, an abundant dietary component is a potent anti-leishmanial agent that acts by inducing topoisomerase II-mediated kinetoplast DNA cleavage leading to apoptosis. Molecular medicine (Cambridge, Mass.), 6(6), 527–541. https://doi.org/10.1007/bf03401792
Tsilioni, I., Taliou, A., Francis, K., & Theoharides, T. C. (2015). Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Translational Psychiatry, 5(9), e647–e655. https://doi.org/10.1038/tp.2015.142
Lin, L. C., Pai, Y. F., & Tsai, T. H. (2015). Isolation of luteolin and luteolin-7-O-glucoside from Dendranthema morifolium Ramat Tzvel and their pharmacokinetics in rats. Journal of Agricultural and Food Chemistry, 63(35), 7700–7706. https://doi.org/10.1021/jf505848z
Dang, H., Meng, M. H. W., Zhao, H., Iqbal, J., Dai, R., Deng, Y., & Lv, F. (2014). Luteolin-loaded solid lipid nanoparticles synthesis, characterization, & improvement of bioavailability, pharmacokinetics in vitro and vivo studies. Journal of Nanoparticle Research, 16(4), 2347. https://doi.org/10.1007/s11051-014-2347-9
Sinha, A., & Suresh, P. K. (2019). Enhanced Induction of apoptosis in HaCaT cells by luteolin encapsulated in PEGylated liposomes—role of caspase-3/caspase-14. Applied Biochemistry and Biotechnology, 188(1), 147–164. https://doi.org/10.1007/s12010-018-2907-z
Shinde, P., Agraval, H., Singh, A., Yadav, U. C. S., & Kumar, U. (2019). Synthesis of luteolin loaded zein nanoparticles for targeted cancer therapy improving bioavailability and efficacy. Journal of Drug Delivery Science and Technology, 52, 369–378. https://doi.org/10.1016/j.jddst.2019.04.044
Acknowledgements
The authors are grateful to Dr. Natesan Pazhanivel for his inputs regarding the histopathological examination of the liver.
Author information
Authors and Affiliations
Contributions
Syed Ilyas Shehnaz (S. I. S.) conceived and designed the experiments, guided by Anitha Roy. S. I. S. performed the experiments, guided by Rajagopalan Vijayaraghavan (R. V.). S. I. S. and R. V. performed the statistical analysis. The first draft of the manuscript was written by S. I. S. with contributions to the methodology section by R. V. and Senthilkumar Sivanesan. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Ethics approval in handling the rats and conducting the animal studies was obtained from the Institutional Animal Ethics Committee of Saveetha Institute of Medical and Technical Sciences (SIMATS) with an approval reference number of (SU/CLAR/RD/007/2019, dated 21 December 2019).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shehnaz, S.I., Roy, A., Vijayaraghavan, R. et al. Luteolin Mitigates Diabetic Dyslipidemia in Rats by Modulating ACAT-2, PPARα, SREBP-2 Proteins, and Oxidative Stress. Appl Biochem Biotechnol 195, 4893–4914 (2023). https://doi.org/10.1007/s12010-023-04544-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04544-4