Abstract
Background and objective
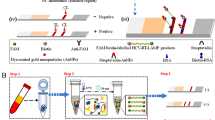
The utilization of direct amplification of nucleic acid from lysate has attracted interest in the advancement of straightforward and economical point-of-care assays. Consequently, this study primarily focuses on the development of a rapid, precise, and cost-effective lateral flow biosensor for the convenient detection of HBV nucleic acid at the point-of-care. Furthermore, the study evaluates the effectiveness of the direct amplification method in comparison to purified nucleic acid samples within the context of LAMP-LF biosensing approaches.
Methods
The experiments conducted in this study utilized clinical serum samples that were confirmed as HBV-positive through real-time PCR assays. Sample preparation involved employing spin column nucleic acid purification and serum heat treatment. To amplify a 250 bp fragment of the HBV polymerase gene, three pairs of specific LAMP primers were utilized, which were biotin-labeled and FITC-labeled for detection purposes. Various incubation temperatures (ranging from 64 to 68 °C) and durations (30 min, 45 min, and 1 h) were investigated to determine the optimal conditions for the LAMP assay. The results were subsequently assessed through fluorometric analysis, white turbidity measurements, and lateral flow assay. Milenia HybriDetect1 strips, designed for immediate use, were employed to visualize the LAMP amplicons. Furthermore, the performance of the lateral flow biosensor was evaluated using 10-fold serial dilutions of a secondary standard containing a viral load of 108 IU/ml.
Results
The optimization of the LAMP reaction was achieved at a temperature of 67 °C, resulting in significant turbidity after a 30-minute incubation period. When the spin column purification method was employed, varying test bands were observed for templates ranging from 108 IU/ml to 101 IU/ml viral load. However, when serum samples underwent heat treatment and the resulting supernatant was directly used for LAMP, the lateral flow assay was capable of detecting a minimum viral load of 103 IU/ml.
Conclusion
In resource-limited settings, the LAMP-LF assay presents a promising solution for HBV testing. However, it is important to note that direct amplification without DNA purification may diminish the performance of the approach.



Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
WHO (2017) Global Hepatitis Report. https://www.who.int/publications/i/item/9789241565455.
Datta S, Chatterjee S, Veer V, Chakravarty R (2012) Molecular biology of the hepatitis B virus for clinicians. J Clin Exp Hepatol 2:353–65
Hellard ME, Chou R, Easterbrook P (2017) WHO guidelines on testing for hepatitis B and C – meeting targets for testing. BMC Infect Dis 17:703. https://doi.org/10.1186/s12879-017-2765-2
Clements CJ, Baoping Y, Crouch A, Hipgrave D, Mansoor O, Nelson CB et al (2006) Progress in the control of hepatitis B infection in the Western Pacific Region. Vaccine 24:1975–82
Busch MP, Should, (2004) HBV DNA NAT replace HBsAg and/or anti-HBc screening of blood donors. Transfus Clin Biol 11:26–32
Datta S, Chatterjee S, Veer V (2014) Recent advances in molecular diagnostics of hepatitis B virus. World J Gastroenterol 20:14615–25
Kuhns MC, Busch MP (2006) New strategies for blood donor screening for hepatitis B virus: nucleic acid testing versus immunoassay methods. Mol Diagn Ther 10:77–91
Xi D, Luo X, Ning Q (2007) Detection of HBV and HCV coinfection by TEM with Au nanoparticle gene probes. J Huazhong Univ Sci Technolog Med Sci 27:532–4
Shevtsov M, Zhao L, Protzer U, van de Klundert MAA (2017) Applicability of metal nanoparticles in the detection and monitoring of hepatitis B virus infection. Viruses 9:E193
Martiskainen I, Talha SM, Vuorenpää K, Salminen T, Juntunen E, Chattopadhyay S et al (2021) Upconverting nanoparticle reporter-based highly sensitive rapid lateral flow immunoassay for hepatitis B virus surface antigen. Anal Bioanal Chem 413:967–78
Gong Y, Zheng Y, Jin B, You M, Wang J, Li X et al (2019) A portable and universal upconversion nanoparticle-based lateral flow assay platform for point-of-care testing. Talanta 201:126–33
Zhao N, Liu JX, Li D, Sun DX (2016) Loop-mediated isothermal amplification for visual detection of hepatitis B virus. Zhonghua Gan Zang Bing Za Zhi 24:406–11
Xiao Y, Thompson AJ, Howell J (2020) Point-of-care tests for hepatitis B: an overview. Cells 9:2233
Chen H-W, Belinskaya T, Zhang Z, Ching W-M (2019) Simple detection of hepatitis B virus in using loop-mediated isothermal amplification method. Mil Med 184:e275-80
Quoc NB, Phuong NDN, Chau NNB, Linh DTP (2018) Closed tube loop-mediated isothermal amplification assay for rapid detection of hepatitis B virus in human blood. Heliyon 4:561
Cai T, Lou G, Yang J, Xu D, Meng Z (2008) Development and evaluation of real-time loop-mediated isothermal amplification for hepatitis B virus DNA quantification: a new tool for HBV management. J Clin Virol 41 4:270–276
Witkowska McConnell W, Davis C, Sabir SR, Garrett A, Bradley-Stewart A, Jajesniak P et al (2021) Paper microfluidic implementation of loop mediated isothermal amplification for early diagnosis of hepatitis C virus. Nat Commun 12:6994
Reboud J, Xu G, Garrett A, Adriko M, Yang Z, Tukahebwa EM et al (2019) Paper-based microfluidics for DNA diagnostics of malaria in low resource underserved rural communities. Proc Natl Acad Sci 116:4834–42
Guo X, Khalid MA, Domingos I, Michala AL, Adriko M, Rowel C et al (2021) Smartphone-based DNA diagnostics for malaria detection using deep learning for local decision support and blockchain technology for security. Nat Electron 4:615–624
Akram A, Islam SMR, Munshi SU, Tabassum S (2018) Detection of hepatitis B virus DNA among chronic and potential occult HBV patients in resource-limited settings by loop-mediated isothermal amplification assay. J Viral Hepat 25:1306–11
Nyan D-C, Ulitzky LE, Cehan N, Williamson P, Winkelman V, Rios M et al (2014) Rapid detection of hepatitis B virus in blood plasma by a specific and sensitive loop-mediated isothermal amplification assay. Clin Infect Dis 59:16–23
New england biolabs inc (2023) NEB LAMP Primer Design Tool version 1.3.0. https://lamp.neb.com/#!/.
Satoh K, Iwata-Takakura A, Yoshikawa A, Gotanda Y, Tanaka T, Yamaguchi T et al (2008) A new method of concentrating hepatitis B virus (HBV) DNA and HBV surface antigen: an application of the method to the detection of occult HBV infection. Vox Sang 95:174–80
Jani I (2022) Peter TF nucleic acid Point-of-care testing to improve diagnostic preparedness. Clin Infect Dis 75:723–8
Hu J, Xiao K, Jin B, Zheng X, Ji F, Bai D (2019) Paper-based point-of-care test with xeno nucleic acid probes. Biotechnol Bioen 116:2764–77
Maity SN, Poonati R, Punati RD, Mallepaddi P, Marothi Y, Mallepaddi PC (2022) Development of sensitive and specific loop-mediated isothermal amplification combined with lateral flow device for the rapid detection of hepatitis B virus infection. Braz J Microbiol 53:615–23
Land KJ, Boeras DI, Chen X-S, Ramsay AR, Peeling RW (2019) REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat Microbiol 4:46–54
Vanhomwegen J, Kwasiborski A, Diop A, Boizeau L, Hoinard D, Vray M et al (2021) Development and clinical validation of loop-mediated isothermal amplification (LAMP) assay to diagnose high HBV DNA levels in resource-limited settings. Clin Microbiol Infect 27(12):1858-e9
Shirato K (2019) Detecting amplicons of loop-mediated isothermal amplification. Microbiol Immunol 63:407–12
Zhou Y, Wu Y, Ding L, Huang X, Xiong Y (2021) Point-of-care COVID-19 diagnostics powered by lateral flow assay. Trends Analyt Chem 145:116
Funding
This study was supported by Istanbul Gelisim University Office of Scientific Research Projects, Grant No.: KAP-050421-AHH.
Author information
Authors and Affiliations
Contributions
AAH conceived the study, planned and performed the experiments, analyzed the data, and supervised the project. AHH and SYB drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
there are no conflicts of interest to declare.
Ethical approval
All participants were provided with informed consent and the study protocol has been approved by the Ethics Committee of the Faculty of medicine, Marmara University in Istanbul, Türkiye (07.05.2021 date & protocol no: 09.2021.577).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Husseini, A.A., Baydar, S.Y. Optimization of a rapid and sensitive nucleic acid lateral flow biosensor for hepatitis B virus detection. Mol Biol Rep 50, 8329–8336 (2023). https://doi.org/10.1007/s11033-023-08730-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08730-9