Abstract
Background
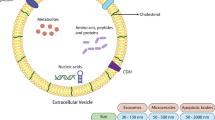
Bacterial outer membrane vesicles have gained increasing attention for its antitumor effect and application in drug delivery. However, the bacterial membrane vesicles (MVs) that are secreted by Gram-positive bacteria are rarely mentioned. Bifidobacterium has a certain anti-tumor effect, but there is a certain risk when injected into human body. Here we investigated the potential of Bifidobacterium-derived membrane vesicles (B-MVs) as therapeutic agents to treat triple-negative breast cancer.
Methods and results
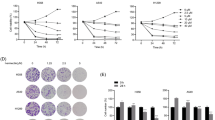
Firstly, we discovered that Bifidobacterium can produce B-MVs and isolated them. In vivo, we found that B-MVs can inhibit tumor growth in mice and the mice were in good state. H&E staining displayed extensive apoptotic cells in tumor tissues. Western blotting and immunohistochemistry showed that B-MVs increased the expression of Bax, while decreased the expression of Bcl-2. These results suggested that B-MVs may induce apoptosis of tumor cells in vivo. Furthermore, to further confirm this phenomenon, we conducted experiments in vitro. Hoechst 33,258 staining assay, flow cytometry and western blotting also demonstrated B-MVs promoted cell apoptosis in vitro.
Conclusions
We speculate B-MVs may inhibit tumor growth by inducing tumor cell apoptosis in triple-negative breast cancer, which provided a new direction in the treatment of TNBC.
Graphical abstract





Similar content being viewed by others
Data availability
The data presented in this study are available on request from the corresponding author.
Abbreviations
- MVs:
-
Membrane vesicles
- OMVs:
-
Outer membrane vesicles
- B-MVs:
-
Bifidobacterium-derived membrane vesicles
- TNBC:
-
Triple-negative breast cancer
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- HER-2:
-
Human epidermal growth factor receptor 2
- V. cholera :
-
Vibrio cholera
- TEM:
-
Transmission electron microscope
- NTA:
-
Nanoparticle tracking analysis
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72(1):7–33. https://doi.org/10.3322/caac.21708
Hu J, Zhang Y, Jiang X et al (2019) ROS-mediated activation and mitochondrial translocation of CaMKII contributes to Drp1-dependent mitochondrial fission and apoptosis in triple-negative breast cancer cells by isorhamnetin and chloroquine. J Exp Clin Cancer Res 38(1):225. https://doi.org/10.1186/s13046-019-1201-4
Yin L, Duan JJ, Bian XW, Yu SC (2020) Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res 22(1):61. https://doi.org/10.1186/s13058-020-01296-5
Fadeel B, Orrenius S (2005) Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Intern Med 258(6):479–517. https://doi.org/10.1111/j.1365-2796.2005.01570.x
Lin X, Wei J, Chen Y et al (2016) Isoorientin from Gypsophila elegans induces apoptosis in liver cancer cells via mitochondrial-mediated pathway. J Ethnopharmacol 187:187–194. https://doi.org/10.1016/j.jep.2016.04.050
Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N (2014) Apoptosis and molecular targeting therapy in cancer. Biomed Res Int 2014:150845. https://doi.org/10.1155/2014/150845
Theron KE, Penny CB, Hosie MJ (2013) The Bax/Bcl-2 apoptotic pathway is not responsible for the increase in apoptosis in the RU486-treated rat uterus during early pregnancy. Reprod Biol 13(4):290–297. https://doi.org/10.1016/j.repbio.2013.09.002
Frion-Herrera Y, Diaz-Garcia A, Ruiz-Fuentes J, Rodriguez-Sanchez H, Sforcin JM (2015) Brazilian green propolis induced apoptosis in human lung cancer A549 cells through mitochondrial-mediated pathway. J Pharm Pharmacol 67(10):1448–1456. https://doi.org/10.1111/jphp.12449
Kashyap D, Garg VK, Goel N (2021) Intrinsic and extrinsic pathways of apoptosis: role in cancer development and prognosis. Adv Protein Chem Struct Biol 125:73–120. https://doi.org/10.1016/bs.apcsb.2021.01.003
Ma ZJ, Lu L, Yang JJ et al (2018) Lariciresinol induces apoptosis in HepG2 cells via mitochondrial-mediated apoptosis pathway. Eur J Pharmacol 821:1–10. https://doi.org/10.1016/j.ejphar.2017.12.027
Abdolalipour E, Mahooti M, Salehzadeh A et al (2020) Evaluation of the antitumor immune responses of probiotic Bifidobacterium bifidum in human papillomavirus-induced tumor model. Microb Pathog 145:104207. https://doi.org/10.1016/j.micpath.2020.104207
Badgeley A, Anwar H, Modi K, Murphy P, Lakshmikuttyamma A (2021) Effect of probiotics and gut microbiota on anti-cancer drugs: mechanistic perspectives. Biochim Biophys Acta Rev Cancer 1875:188494. https://doi.org/10.1016/j.bbcan.2020.188494
Li M, Zhou H, Yang C et al (2020) Bacterial outer membrane vesicles as a platform for biomedical applications: an update. J Control Release 323:253–268. https://doi.org/10.1016/j.jconrel.2020.04.031
Sartorio MG, Pardue EJ, Feldman MF, Haurat MF (2021) Bacterial outer membrane vesicles: from discovery to applications. Annu Rev Microbiol 75:609–630. https://doi.org/10.1146/annurev-micro-052821-031444
Aly RG, El-Enbaawy MI, Abd El-Rahman SS, Ata NS (2021) Antineoplastic activity of Salmonella typhimurium outer membrane nanovesicles. Exp Cell Res 399(1):112423. https://doi.org/10.1016/j.yexcr.2020.112423
Jin L, Zhang Z, Tan X et al (2022) Antitumor effect of Escherichia coli-derived outer membrane vesicles on neuroblastoma in vitro and in vivo. Acta Biochim Biophys Sin (Shanghai) 54(9):1301–1313. https://doi.org/10.3724/abbs.2022127
Cui C, Guo T, Zhang S et al (2022) Bacteria-derived outer membrane vesicles engineered with over-expressed pre-miRNA as delivery nanocarriers for cancer therapy. Nanomedicine 45:102585. https://doi.org/10.1016/j.nano.2022.102585
Kuerban K, Gao X, Zhang H et al (2020) Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm Sin B 10(8):1534–1548. https://doi.org/10.1016/j.apsb.2020.02.002
Li Y, Ma X, Yue Y et al (2022) Rapid surface display of mRNA antigens by bacteria-derived outer membrane vesicles for a personalized tumor vaccine. Adv Mater 34(20):e2109984. https://doi.org/10.1002/adma.202109984
Zhuang Q, Xu J, Deng D et al (2021) Bacteria-derived membrane vesicles to advance targeted photothermal tumor ablation. Biomaterials. 268:120550. https://doi.org/10.1016/j.biomaterials.2020.120550
Yin X, Yu B, Tang Z et al (2013) Bifidobacterium infantis-mediated HSV-TK/GCV suicide gene therapy induces both extrinsic and intrinsic apoptosis in a rat model of bladder cancer. Cancer Gene Ther 20(2):77–81. https://doi.org/10.1038/cgt.2012.86
Eslami M, Yousefi B, Kokhaei P et al (2019) Importance of probiotics in the prevention and treatment of colorectal cancer. J Cell Physiol 234(10):17127–17143. https://doi.org/10.1002/jcp.28473
Lee DK, Jang S, Kim MJ et al (2008) Anti-proliferative effects of Bifidobacterium adolescentis SPM0212 extract on human colon cancer cell lines. BMC Cancer. https://doi.org/10.1186/1471-2407-8-310
Kim S, Lee HH, Choi W et al (2022) Anti-tumor effect of heat-killed Bifidobacterium bifidum on human gastric cancer through Akt-p53-dependent mitochondrial apoptosis in Xenograft models. Int J Mol Sci. https://doi.org/10.3390/ijms23179788
Lee SH, Cho SY, Yoon Y et al (2021) Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice. Nat Microbiol 6(3):277–288. https://doi.org/10.1038/s41564-020-00831-6
Li Q, Li Y, Wang Y et al (2021) Oral administration of Bifidobacterium breve promotes antitumor efficacy via dendritic cells-derived interleukin 12. Oncoimmunology 10(1):1868122. https://doi.org/10.1080/2162402X.2020.1868122
Shi Y, Zheng W, Yang K et al (2020) Intratumoral accumulation of gut microbiota facilitates CD47-based immunotherapy via STING signaling. J Exp Med. https://doi.org/10.1084/jem.20192282
Huang X, Pan J, Xu F et al (2021) Bacteria-based cancer immunotherapy. Adv Sci (Weinh) 8(7):2003572. https://doi.org/10.1002/advs.202003572
Duong MT, Qin Y, You SH, Min JJ (2019) Bacteria-cancer interactions: bacteria-based cancer therapy. Exp Mol Med 51(12):1–15. https://doi.org/10.1038/s12276-019-0297-0
Galloway-Pena J, Brumlow C, Shelburne S (2017) Impact of the microbiota on bacterial infections during cancer treatment. Trends Microbiol 25(12):992–1004. https://doi.org/10.1016/j.tim.2017.06.006
Yu YJ, Wang XH, Fan GC (2018) Versatile effects of bacterium-released membrane vesicles on mammalian cells and infectious/inflammatory diseases. Acta Pharmacol Sin 39(4):514–533. https://doi.org/10.1038/aps.2017.82
Kulp A, Kuehn MJ (2010) Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol 64(1):163–184. https://doi.org/10.1146/annurev.micro.091208.073413
Schwechheimer C, Kuehn MJ (2015) Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol 13(10):605–619. https://doi.org/10.1038/nrmicro3525
Wang D, Liu C, You S et al (2020) Bacterial vesicle-cancer cell hybrid membrane-coated nanoparticles for tumor specific immune activation and photothermal therapy. ACS Appl Mater Interfaces 12(37):41138–41147. https://doi.org/10.1021/acsami.0c13169
Chen Q, Bai H, Wu W et al (2020) Bioengineering bacterial vesicle-coated polymeric nanomedicine for enhanced cancer immunotherapy and metastasis prevention. Nano Lett 20(1):11–21. https://doi.org/10.1021/acs.nanolett.9b02182
Qing S, Lyu C, Zhu L et al (2020) Biomineralized bacterial outer membrane vesicles potentiate safe and efficient tumor microenvironment reprogramming for anticancer therapy. Adv Mater 32(47):e2002085. https://doi.org/10.1002/adma.202002085
Huang W, Zhang Q, Li W et al (2020) Development of novel nanoantibiotics using an outer membrane vesicle-based drug efflux mechanism. J Control Release 317:1–22. https://doi.org/10.1016/j.jconrel.2019.11.017
Alves NJ, Turner KB, Medintz IL, Walper SA (2015) Emerging therapeutic delivery capabilities and challenges utilizing enzyme/protein packaged bacterial vesicles. Ther Deliv 6(7):873–887. https://doi.org/10.4155/tde.15.40
Huang W, Shu C, Hua L et al (2020) Modified bacterial outer membrane vesicles induce autoantibodies for tumor therapy. Acta Biomater 108:300–312. https://doi.org/10.1016/j.actbio.2020.03.030
Chen Y, Wu H, Wang X et al (2018) Huaier Granule extract inhibit the proliferation and metastasis of lung cancer cells through down-regulation of MTDH, JAK2/STAT3 and MAPK signaling pathways. Biomed Pharmacother 101:311–321. https://doi.org/10.1016/j.biopha.2018.02.028
Li D, Wen S (2020) Silencing of lncRNA LINC00346 inhibits the proliferation and promotes the apoptosis of colorectal cancer cells through inhibiting JAK1/STAT3 signaling. Cancer Manag Res 12:4605–4614. https://doi.org/10.2147/CMAR.S249491
Adams JM, Cory S (2007) Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol 19(5):488–496. https://doi.org/10.1016/j.coi.2007.05.004
Burke PJ (2017) Mitochondria, bioenergetics and apoptosis in cancer. Trends Cancer 3(12):857–870. https://doi.org/10.1016/j.trecan.2017.10.006
Acknowledgements
The authors would like to thank the Chongqing Medical University, State Key Laboratory of Ultrasound in Medicine and Engineering for providing the necessary facilities to conduct this study.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: YJ. Methodology: YJ, LW, BY, GM, ZC, JM and XC. Software: YJ. Writing—original draft preparation: YJ. Writing—review and editing: YJ. Supervision: LF and ZW.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
All the experimental procedures were approved by the animal ethics committee of Chongqing Medical University (Date 2022.6.28/No2022155). All procedures involving animals were conducted with the guidelines of the Institutional Animal Care and Use Committee of Chongqing Medical University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, Y., Wang, L., Yang, B. et al. Bifidobacterium-derived membrane vesicles inhibit triple-negative breast cancer growth by inducing tumor cell apoptosis. Mol Biol Rep 50, 7547–7556 (2023). https://doi.org/10.1007/s11033-023-08702-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08702-z