Abstract
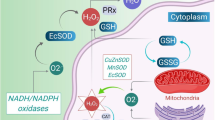
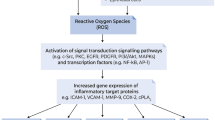
Background:Asthma is a prolonged inflammatory disorder of the airways, that affects an estimated 300 million people worldwide. Asthma is triggered by numerous endogenous and exogenous stimuli with symptoms like wheezing, cough, short of breath, chest tightening, airway obstruction, and hyperreactivity observed in patients. Objective: The review seeks to identify targets of redox imbalance and inflammation that could be explored to create effective treatments for asthma. Methods: The methodology involved a search and review of literature relating to asthma pathogenesis, redox homeostasis, and inflammation. Results: Eosinophils and neutrophils are involved in asthma pathogenesis. These inflammatory cells generate high levels of endogenous oxidants such as hydrogen peroxide and superoxide, which could result in redox imbalance in the airways of asthmatics. Redox imbalance occurs when the antioxidant systems becomes overwhelmed resulting in oxidative stress. Oxidative stress and inflammation have been linked with asthma inflammation and severity. Reactive oxygen species (ROS)/reactive nitrogen species (RNS) cause lung inflammation by activating nuclear factor kappa-B (NF-κB), mitogen-activated protein kinase (MAPK), activator protein-1, as well as additional transcription factors. These factors stimulate cytokine production which ultimately activates inflammatory cells in the bronchi, causing lung cellular injury and destruction. ROS/RNS is also produced by these inflammatory cells to eradicate invading bacteria. Antioxidant treatments for asthma have not yet been fully explored. Conclusion: Redox and inflammatory processes are viable targets that could be explored to create better therapy for asthma.




Similar content being viewed by others
Data availability
Not applicable.
Code availability
None.
References
Mathis BJ, Kusumoto M, Zaboronok A, Hiramatsu Y (2022) Packaging and delivery of asthma therapeutics. Pharmaceutics 14(1):92. https://doi.org/10.3390/pharmaceutics14010092
Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, Bhutta ZA (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet 396:1204–1222. https://doi.org/10.1016/S0140-6736(20)30925-9
Ojo OT, Ajibare AO, Odeyemi A, Fapohunda T, Adeyeye OO (2023) Clinical utility of peak flow meter in asthma diagnosis and monitoring in low-and middle-income countries: a narrative review. Int J Med Health Dev. https://doi.org/10.4103/ijmh.IJMH_4_23
Ortega H, Nickle D, Carter L (2021) Rhinovirus and asthma: challenges and opportunities. Rev Med Virol 31(4):e2193. https://doi.org/10.1002/rmv.2193
Albano GD, Gagliardo RP, Montalbano AM, Profita M (2022) Overview of the mechanisms of oxidative stress: impact in inflammation of the airway diseases. Antioxidants 11(11):2237. https://doi.org/10.3390/antiox11112237
Leiba J, Özbilgiç R, Hernández L, Demou M, Lutfalla G, Yatime L, Nguyen-Chi M (2023) Molecular actors of inflammation and their signaling pathways: mechanistic insights from zebrafish. Biology 12(2):153. https://doi.org/10.3390/biology12020153
Singh S, Dutta J, Ray A, Karmakar A, Mabalirajan U (2023) Airway epithelium: a neglected but crucial cell type in asthma pathobiology. Diagnostics 13(4):808. https://doi.org/10.3390/diagnostics13040808
Kuruvilla ME, Lee FE, Lee GB (2019) Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol 56(2):219–233. https://doi.org/10.1007/s12016-018-8712-1
Amaral-Machado L, Oliveira WN, Moreira-Oliveira SS, Pereira DT, Alencar ÉN, Tsapis N, Egito E (2020) Use of natural products in asthma treatment. Evid Based Complement Altern Med: eCAM 2020:1021258. https://doi.org/10.1155/2020/1021258
Checa J, Aran JM (2020) Airway redox homeostasis and inflammation gone awry: from molecular pathogenesis to emerging therapeutics in respiratory pathology. Int J Mol Sci 21(23):9317. https://doi.org/10.3390/ijms21239317
Ramakrishnan M, Papolu PK, Satish L, Vinod KK, Wei Q, Sharma A, Zhou M (2022) Redox status of the plant cell determines epigenetic modifications under abiotic stress conditions and during developmental processes. J Adv Res 42:99–116. https://doi.org/10.1016/j.jare.2022.04.007
Michaeloudes C, Abubakar-Waziri H, Lakhdar R, Raby K, Dixey P, Adcock IM, Chung KF (2022) Molecular mechanisms of oxidative stress in asthma. Mol Asp Med 85:101026. https://doi.org/10.1016/j.mam.2021.101026
Qu J, Li Y, Zhong W, Gao P, Hu C (2017) Recent developments in the role of reactive oxygen species in allergic asthma. J Thorac Dis 9(1):32–43. https://doi.org/10.21037/jtd.2017.01.05
Comhair SA, Erzurum SC (2010) Redox control of asthma: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal 12(1):93–124. https://doi.org/10.1089/ars.2008.2425
Hayyan M, Hashim MA, AlNashef IM (2016) Superoxide ion: generation and chemical implications. Chem Rev 116(5):3029–3085. https://doi.org/10.1021/acs.chemrev.5b00407
Sahiner UM, Birben E, Erzurum S, Sackesen C, Kalayci O (2018) Oxidative stress in asthma: part of the puzzle. Pediatr Allergy Immunol. https://doi.org/10.1111/pai.12965
Neves B, Pérez-Sala D, Ferreira HB, Guerra IM, Moreira AS, Domingues P, Melo T (2022) Understanding the nitrolipidome: From chemistry to mass spectrometry and biological significance of modified complex lipids. Prog Lipid Res. https://doi.org/10.1016/j.plipres.2022.101176
Phaniendra A, Jestadi DB, Periyasamy L (2015) Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem: IJCB 30(1):11–26. https://doi.org/10.1007/s12291-014-0446-0
Alugoju P, Tencomnao T (2023) Production and role of plants secondary metabolites under various environmental pollution. In: plants and their interaction to environmental pollution, Elsevier, pp. 379–410, https://doi.org/10.1016/B978-0-323-99978-6.00018-2
Liwsrisakun C, Chaiwong W, Bumroongkit C, Deesomchok A, Theerakittikul T, Limsukon A, Trongtrakul K, Tajarernmuang P, Niyatiwatchanchai N, Pothirat C (2023) Influence of particulate matter on asthma control in adult asthma. Atmosphere 14(2):410. https://doi.org/10.3390/atmos14020410
Dhital NB, Wang SX, Lee CH, Su J, Tsai MY, Jhou YJ, Yang HH (2021) Effects of driving behavior on real-world emissions of particulate matter, gaseous pollutants and particle-bound PAHs for diesel trucks. Environ Pollut 286:117292. https://doi.org/10.1016/j.envpol.2021.117292
Wang B, Lau YS, Huang Y, Organ B, Chuang HC, Ho SSH, Ho KF (2021) Chemical and toxicological characterization of particulate emissions from diesel vehicles. J Hazard Mater 405:124613. https://doi.org/10.1016/j.jhazmat.2020.124613
Lugg ST, Scott A, Parekh D, Naidu B, Thickett DR (2022) Cigarette smoke exposure and alveolar macrophages: mechanisms for lung disease. Thorax 77(1):94–101. https://doi.org/10.1136/thoraxjnl-2020-216296
Thimmulappa RK, Chattopadhyay I, Rajasekaran S (2020) Oxidative stress mechanisms in the pathogenesis of environmental lung diseases. Oxid Stress Lung Dis 2:103–137. https://doi.org/10.1007/978-981-32-9366-3_5
Kleniewska P, Pawliczak R (2019) The influence of apocynin, lipoic acid and probiotics on antioxidant enzyme levels in the pulmonary tissues of obese asthmatic mice. Life Sci 234:116780. https://doi.org/10.1016/j.lfs.2019.116780
Wang Y, Branicky R, Noë A, Hekimi S (2018) Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol 217(6):1915–1928. https://doi.org/10.1083/jcb.201708007
Leikauf GD, Kim SH, Jang AS (2020) Mechanisms of ultrafine particle-induced respiratory health effects. Exp Mol Med 52(3):329–337. https://doi.org/10.1038/s12276-020-0394-0
Kumari M, Raj R, Kumar S, Srivastava A, Gond DP (2021) Azadirachta indica attenuates airway inflammation and oxidative stress in the asthmatic mice. J Pharmacognosy Phytochem 10(1):1777–1785. https://doi.org/10.22271/phyto.2021.v10.i1y.13608
Cellat M, Kuzu M, İşler CT, Etyemez M, Dikmen N, Uyar A, Güvenç M (2021) Tyrosol improves ovalbumin (OVA)-induced asthma in rat model through prevention of airway inflammation. Naunyn-Schmiedeberg’s Arch Pharmacol 394(10):2061–2075. https://doi.org/10.21203/rs.3.rs-447166/v1
Karadogan B, Beyaz S, Gelincik A, Buyukozturk S, Arda N (2022) Evaluation of oxidative stress biomarkers and antioxidant parameters in allergic asthma patients with different level of asthma control. J Asthma 59(4):663–672. https://doi.org/10.1080/02770903.2020.1870129
Oudjedi A, Aissa KS (2020) Associations between obesity, asthma and physical activity in children and adolescents. Apunts Sports Med 55(205):39–48. https://doi.org/10.1016/j.apunsm.2020.02.003
Serra MF, Cotias AC, Pimentel AS, Arantes ACSD, Pires ALA, Lanzetti M, Martins MA (2022) Gold nanoparticles inhibit steroid-insensitive asthma in mice preserving histone deacetylase 2 and NRF2 pathways. Antioxidants 11(9):1659. https://doi.org/10.3390/antiox11091659
Sahiner UM, Birben E, Erzurum S, Sackesen C, Kalayci O (2011) Oxidative stress in asthma. Shayga 4(10):151–158. https://doi.org/10.1097/wox.0b013e318232389e
Crinnion WJ (2012) Do environmental toxicants contribute to allergy and asthma?. Altern Med Rev 17:6–18
Ferrini ME, Simons BJ, Bassett DJ, Bradley MO, Roberts K, Jaffar Z (2013) S-nitrosoglutathione reductase inhibition regulates allergen-induced lung inflammation and airway hyperreactivity. PLoS ONE 8(7):e70351. https://doi.org/10.1371/journal.pone.0070351
Lee IT, Yang CM (2012) Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem Pharmacol 84:581–590
Blaner WS, Shmarakov IO, Traber MG (2021) Vitamin A and vitamin E: will the real antioxidant please stand up? Annu Rev Nutr 41:105–131. https://doi.org/10.1146/annurev-nutr-082018-124228
Gambaro RC, Seoane A, Padula G (2023) Vitamin E protective effects on genomic and cellular damage caused by paediatric preventive supplementation for anaemia: an experimental model. Br J Nutr 129(3):468–477. https://doi.org/10.1017/S0007114522001556
Peh HY, Ho WE, Cheng C, Chan TK, Seow AC, Lim AY, Fong CW, Seng KY, Ong CN, Wong WS (2015) Vitamin E isoform γ-tocotrienol downregulates house dust mite-induced asthma. J Immunol 195(2):437–444. https://doi.org/10.4049/jimmunol.1500362
Harada T, Yamasaki A, Chikumi H, Hashimoto K, Okazaki R, Takata M, Fukushima T, Watanabe M, Kurai J, Halayko AJ, Shimizu E (2015) γ-Tocotrienol reduces human airway smooth muscle cell proliferation and migration. Pulm Pharmacol Ther 32:45–52. https://doi.org/10.1016/j.pupt.2015.04.003
Aktar MW (2016) Antioxidants: chemistry and their impact on health. Pesticide Residue Laboratory, Department of Agricultural Chemicals, Bidhan Chandra Krishi Viswavidyalaya, Mohanpur-741252, Nadia, West Bengal, Ind iawww. shamskm. com
Nakai K, Tsuruta D (2021) What are reactive oxygen species, free radicals, and oxidative stress in skin diseases? Int J Mol Sci 22(19):10799. https://doi.org/10.3390/ijms221910799
Bansal P, Saw S, Govindaraj D, Arora N (2014) Intranasal administration of a combination of choline chloride, vitamin C, and selenium attenuates the allergic effect in a mouse model of airway disease. Free Radic Biol Med 73:358–365. https://doi.org/10.1016/j.freeradbiomed.2014.05.018
Zhu LY, Ni ZH, Luo XM, Wang XB (2017) Advance of antioxidants in asthma treatment. World J Respirol 7(1):17–28. https://doi.org/10.5320/wjr.v7.i1.17
Sussan TE, Gajghate S, Chatterjee S, Mandke P, McCormick S, Sudini K, Kumar S, Breysse PN, Diette GB, Sidhaye VK (2015) Nrf2 reduces allergic asthma in mice through enhanced airway epithelial cytoprotective function. Am J Physiol Lung Cell Mol Physiol 309:L27–L36
Lambrecht BN, Hammad H (2012) Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu Rev Immunol 30:243–270
Zaiss DMW, Gause WC, Osborne LC, Artis D (2015) Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity 42(2):216–226. https://doi.org/10.1016/j.immuni.2015.01.020
Chang S, Linderholm A, Franzi L, Kenyon N, Grasberger H, Harper R (2013) Dual oxidase regulates neutrophil recruitment in allergic airways. Free Radical Biol Med 65:38–46. https://doi.org/10.1016/j.freeradbiomed.2013.06.012
Liu H, Zhao Y, Xie A, Kim TY, Terentyeva R, Liu M, Shi G, Feng F, Choi BR, Terentyev D, Hamilton S, Dudley SC Jr (2021) Interleukin-1β, oxidative stress, and abnormal calcium handling mediate diabetic arrhythmic risk. JACC Basic Transl Sci 6(1):42–52. https://doi.org/10.1016/j.jacbts.2020.11.002
Wu L, Pan Y (2019) Reactive oxygen species mediate TNF-α-induced inflammatory response in bone marrow mesenchymal cells. Iran J Basic Med Sci 22(11):1296–1301. https://doi.org/10.22038/ijbms.2019.37893.9006
Aldakheel FM, Thomas PS, Bourke JE, Matheson MC, Dharmage SC, Lowe AJ (2016) Relationships between adult asthma and oxidative stress markers and pH in exhaled breath condensate: a systematic review. Allergy 71:741–757
Mark JC, Ho SP, Ho AS, Law BK, Cheung AH, Ho JC, Ip MS, Chan-Yeung MM (2013) Sustained elevation of systemic oxidative stress and inflammation in exacerbation and remission of asthma. ISRN Allergy 29:561831. https://doi.org/10.1155/2013/561831
Sood A, Qualls C, Seagrave J, McDonald J, Shohreh R, Chiavaroli A, Schuyler M (2013) Effect of allergen inhalation on airway oxidant stress, using exhaled breath condensate 8-isoprostane, in mild asthma. J Asthma 50:449–456
Ökrös Z, Endreffy E, Novak Z, Maroti Z, Monostori P, Varga IS, Király A, Turi S (2012) Changes in NADPH oxidase mRNA level can be detected in blood at inhaled corticosteroid treated asthmatic children. Life Sci 91:907–911
Fatani SH (2014) Biomarkers of oxidative stress in acute and chronic bronchial asthma. J Asthma 51:578–584
Emin O, Hasan A, Rusen DM (2015) Plasma paraoxonase, oxidative status level, and their relationship with asthma control test in children with asthma. Allergol Immunopathol 43:346–352
Wan WY, Hollins F, Haste L, Woodman L, Hirst RA, Bolton S, Gomez E, Sutcliffe A, Desai D, Chachi L (2016) NADPH oxidase-4 overexpression is associated with epithelial ciliary dysfunction in neutrophilic asthma. Chest 149:1445–1459
Sagdic A, Sener O, Bulucu F, Karadurmus N, Özel HE, Yamanel L, Tasci C, Naharci I, Ocal R, Aydin A (2015) Oxidative stress status and plasma trace elements in patients with asthma or allergic rhinitis. Allergol Immunopathol (Madr) 39:200–205
Ben Anes A, Ben Nasr H, Fetoui H, Bchir S, Chahdoura H, Yacoub S, Garrouch A, Benzarti M, Tabka Z, Chahed K (2016) Alteration in systemic markers of oxidative and antioxidative status in Tunisian patients with asthma: relationships with clinical severity and airflow limitation. J Asthma 53:227–237
McKernan DP (2020) Pattern recognition receptors as potential drug targets in inflammatory disorders. Adv Protein Chem Struct Biol 119:65–109. https://doi.org/10.1016/bs.apcsb.2019.09.001
Wicherska-Pawłowska K, Wróbel T, Rybka J (2021) Toll-like receptors (TLRs), NOD-like receptors (NLRs), and RIG-I-like receptors (RLRs) in innate immunity. TLRs, NLRs, and RLRs ligands as immunotherapeutic agents for hematopoietic diseases. Int J Mol Sci 22(24):13397. https://doi.org/10.3390/ijms222413397
Maskrey BH, Megson IL, Whitfield PD, Rossi AG (2011) Mechanisms of resolution of inflammation. Arterioscler Thromb Vasc Biol 31(5):1001–1006
Ortega-Gómez A, Perretti M, Soehnlein O (2013) Resolution of inflammation: an integrated view. EMBO Mol Med 5(5):661–674
Najar M, Krayem M, Merimi M, Burny A, Meuleman N, Bron D, Raicevic G, Lagneaux L (2018) Insights into inflammatory priming of mesenchymal stromal cells: functional biological impacts. Inflamm Res 67:467–477. https://doi.org/10.1007/s00011-018-1131-1
Joseph C, Tatler AL (2022) Pathobiology of airway remodeling in asthma: the emerging role of integrins. J Asthma Allergy. https://doi.org/10.2147/JAA.S267222
Kubo T, Morita H, Sugita K, Akdis CA (2017) Introduction to mechanisms of allergic diseases. In: Masoodi MH, Rehman MU (eds) Middleton’s allergy essentials. Elsevier, Amsterdam, pp 1–27
Matucci A, Bormioli S, Nencini F, Maggi E, Vultaggio A (2021) The emerging role of type 2 inflammation in asthma. Expert Rev Clin Immunol 17(1):63–71. https://doi.org/10.1080/1744666X.2020.1860755
Carr TF, Berdnikovs S, Simon HU, Bochner BS, Rosenwasser LJ (2016) Eosinophilic bioactivities in severe asthma. World Allergy Org J 9(1):21
Martín-Orozco E, Norte-Muñoz M, Martínez-García J (2017) Regulatory T-cells in allergy and asthma. Front Pediatr 5:117. https://doi.org/10.3389/fped.2017.00117
Bakakos A, Loukides S, Bakakos P (2019) Severe eosinophilic asthma. J Clin Med 8:1375. https://doi.org/10.3390/jcm8091375
Bakakos A, Rovina N, Bakakos P (2021) Treatment challenges in severe eosinophilic asthma: differential response to anti-IL-5 and anti-IL-5R therapy. Int J Mol Sci 22(8):3969. https://doi.org/10.3390/ijms22083969
Domingo C (2017) Overlapping effects of new monoclonal antibodies for severe asthma. Drugs 77(16):1769–1787. https://doi.org/10.1007/s40265-017-0810-5
Fettrelet T, Gigon L, Karaulov A, Yousefi S, Simon HU (2021) The enigma of eosinophil degranulation. Int J Mol Sci 22:7091. https://doi.org/10.3390/ijms22137091
Carmo LA, Bonjour K, Ueki S, Neves JS, Liu L, Spencer LA, Dvorak AM, Weller PF, Melo RC (2016) CD63 is tightly associated with intracellular, secretory events chaperoning piecemeal degranulation and compound exocytosis in human eosinophils. J Leukoc Biol 100:391–401
Barnes PJ (2017) Cellular and molecular mechanisms of asthma and COPD. Clin Sci 131(13):1541–1558. https://doi.org/10.1042/CS20160487
Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, Martin RJ, Alam R (2014) Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol 134(5):1175-1186.e7. https://doi.org/10.1016/j.jaci.2014.05.038
Liu W, Liu S, Verma M, Zafar I, Good JT, Rollins D, Groshong S, Gorska MM, Martin RJ, Alam R (2017) Mechanism of TH2/TH17-predominant and neutrophilic TH2/TH17-low subtypes of asthma. J Allergy Clin Immunol 139(5):1548-1558.e4. https://doi.org/10.1016/j.jaci.2016.08.032
Arora P, Ansari SH (2019) Role of various mediators in inflammation of asthmatic airways. Intechopen. https://doi.org/10.5772/intechopen.84357
Wang M, Gao P, Wu X, Chen Y, Feng Y, Yang Q, Xu Y, Zhao J, Xie J (2016) Impaired anti-inflammatory action of glucocorticoid in neutrophil from patients with steroid-resistant asthma. Respir Res 17(1):153. https://doi.org/10.1186/s12931-016-0462-0
Fu JJ, Baines KJ, Wood LG, Gibson PG (2013) Systemic inflammation is associated with differential gene expression and airway neutrophilia in asthma. OMICS: J Integr Biol 17:187–199
Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, Phillips BR, Mauger DT, Comhair SA, Erzurum SC, Johansson MW, Jarjour NN, Coverstone AM, Castro M, Holguin F, Wenzel SE, Woodruff PG, Bleecker ER, Fahy JV, National Heart, Lung, and Blood Institute Severe Asthma Research Program (2016) Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med 4(7):574–584. https://doi.org/10.1016/S2213-2600(16)30048-0
Robinson MB, Deshpande DA, Chou J, Cui W, Smith S, Langefeld C, Hastie AT, Bleecker ER, Hawkins GA (2015) IL-6 trans-signaling increases expression of airways disease genes in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 309(2):L129–L138. https://doi.org/10.1152/ajplung.00288.2014
Zhong J, Shi G (2019) Regulation of inflammation in chronic disease. Front Immunol 10:737
Harb H, Stephen-Victor E, Crestani E, Benamar M, Massoud A, Cui Y, Chatila TA (2020) A regulatory T cell Notch4–GDF15 axis licenses tissue inflammation in asthma. Nature Immunol 21(11):1359–1370
Busse WW (2019) Biological treatments for severe asthma: a major advance in asthma care. Allergol Int 68(2):158–166
Cardenas A, Sordillo JE, Rifas-Shiman SL, Chung W, Liang L, Coull BA, Gold DR (2019) The nasal methylome as a biomarker of asthma and airway inflammation in children. Nat Commun 10(1):1–10
Bergantini L, Cameli P, d’Alessandro M, Vietri L, Perruzza M, Pieroni M, Bargagli E (2020) Regulatory T cells in severe persistent asthma in the era of monoclonal antibodies target therapies. Inflammation 43(2):393–400
Xian M, Feng M, Dong Y, Wei N, Su Q, Li J (2020) Changes in CD4+ CD25+ FoxP3+ regulatory T cells and serum cytokines in sublingual and subcutaneous immunotherapy in allergic rhinitis with or without asthma. Int Arch Allergy Immunol 181(1):71–80
Lin SC, Shi LS, Ye YL (2019) Advanced molecular knowledge of therapeutic drugs and natural products focusing on inflammatory cytokines in asthma. Cells 8(7):685
Lambrecht BN, Hammad H, Fahy JV (2019) The cytokines of asthma. Immunity 50(4):975–991
Gour N, Wills-Karp M (2015) IL-4 and IL-13 signaling in allergic airway disease. Cytokine 75(1):68–78. https://doi.org/10.1016/j.cyto.2015.05.014
Thomson NC, Rubin AS, Niven RM, Corris PA, Siersted HC, Olivenstein R (2017) Long-term (5 year) safety of bronchial thermoplasty: asthma intervention research (AIR) trial. BMC Pulm Med 11(8):143
Brasier AR, Victor S, Ju H, Busse WW, Curran-Everett D, Bleecker E (2020) Predicting intermediate phenotypes in asthma using bronchoalveolar lavage-derived cytokines. Clin Transl Sci 3:147–157
Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H (2019) Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol 127:382–389
Lommatzsch M (2020) Immune modulation in asthma: current concepts and future strategy. Thematic Rev Ser 99(7):566–576
Krug N, Hohlfeld JM, Kirsten AM, Kornmann O, Beeh K, M…. Kappeler, D. (2015) Allergen-induced asthmatic responses modified by a GATA3-specific DNAzyme. N Engl J Med 372(21):1987–1995
McCrae C, Olsson M, Aurell M (2018) On-demand inhaled interferon-beta 1a for the prevention of severe asthma exacerbations: results of the INEXAS phase 2a study. Am J Respir Crit Care Med 197:A6165
Cazzola M, Calzetta L, Rogliani P, Matera MG (2019) Ensifentrine (RPL554): an investigational PDE3/4 inhibitor for the treatment of COPD. Expert Opin Investig Drugs 28(10):827–833
Hanania NA, Wenzel S, Rosén K, Hsieh HJ, Mosesova S, Choy DF, Lal P, Arron JR, Harris JM, Busse W (2013) Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med 187(8):804–811. https://doi.org/10.1164/rccm.201208-1414OC
Holgate ST, Chuchalin AG, Hébert J, Lötvall J, Persson GB, Chung KF, Bousquet J, Kerstjens HA, Fox H, Thirlwell J, Cioppa GD, Omalizumab 011 International Study Group (2004) Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy 34(4):632–638. https://doi.org/10.1111/j.1365-2222.2004.1916.x
Castro M, Rubin AS, Laviolette M, Fiterman J, De Andrade Lima M, Shah PL, Fiss E, Olivenstein R, Thomson NC, Niven RM, Pavord ID, Simoff M, Duhamel DR, McEvoy C, Barbers R, Ten Hacken NH, Wechsler ME, Holmes M, Phillips MJ, Erzurum S, AIR2 Trial Study Group (2010) Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 181(2):116–124. https://doi.org/10.1164/rccm.200903-0354OC
Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, Ortega HG, Pavord ID, SIRIUS Investigators (2014) Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. New Engl J Med 371(13):1189–1197. https://doi.org/10.1056/NEJMoa1403291
Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, Humbert M, Katz LE, Keene ON, Yancey SW, Chanez P (2014) MENSA Investigators
Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, Ortega H, Chanez P (2012) Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 380(9842):651–659. https://doi.org/10.1016/S0140-6736(12)60988-X
Hoy SM (2022) Tezepelumab: first approval. Drugs 82(4):461–468. https://doi.org/10.1007/s40265-022-01679-2
Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, Colice G (2021) Tezepelumab in adults and adolescents with severe, uncontrolled asthma. New Engl J Med 384(19):1800–1809. https://doi.org/10.1056/NEJMoa2034975
Matera MG, Calzetta L, Rinaldi B, Cazzola M (2017) Pharmacokinetic/pharmacodynamic drug evaluation of benralizumab for the treatment of asthma. Expert Opin Drug Metab Toxicol 13(9):1007–1013. https://doi.org/10.1080/17425255.2017.1359253
Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, Pirozzi G, Sutherland ER, Evans RR, Joish VN, Eckert L, Graham NM, Stahl N, Yancopoulos GD, Louis-Tisserand M, Teper A (2016) Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 388(10039):31–44. https://doi.org/10.1016/S0140-6736(16)30307-5
Castro M, Rubin A, Laviolette M, Hanania NA, Armstrong B, Cox G (2011) Persistence of effectiveness of bronchial thermoplasty in patients with severe asthma. Ann Allergy Asthma Immunol 107:65–70
Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, Murphy K, Maspero JF, O’Brien C, Korn S (2015) Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med 3(5):355–366. https://doi.org/10.1016/S2213-2600(15)00042-9
Brightling CE, Brusselle G, Altman P (2019) The impact of the prostaglandin D 2 receptor 2 and its downstream effects on the pathophysiology of asthma. Allergy 75(4):761–768. https://doi.org/10.1111/all.14001
Guillemot-Legris O, Muccioli GG (2021) The oxysterome and its receptors as pharmacological targets in inflammatory diseases. Br J Pharmacol. https://doi.org/10.1111/bph.15479
Acknowledgements
None
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MB. The draft was critically revised by OJA, DER and OSA and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
Not required.
Consent to participate
None.
Consent for publication
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barnabas, M., Awakan, O.J., Rotimi, D.E. et al. Exploring redox imbalance and inflammation for asthma therapy. Mol Biol Rep 50, 7851–7865 (2023). https://doi.org/10.1007/s11033-023-08688-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08688-8