Abstract
Objective
Escherichia coli ST131 is a pandemic clone associated with multidrug resistance, starting with beta-lactamase production and fluoroquinolone resistance in the first place, leading to significant systemic infections. Clones that develop due to the frequency of antimicrobial resistance and the rate of spread in our country are important issues that need to be investigated. This study aims to investigate the incidence of ST131which is a “high-risk pandemic clone E. coli” in ESBL-producing and non-ESBL-producing strains, as well as their biofilm-forming abilities and antibiotic resistance rates.
Materials and methods
A total of 86 E. coli isolates were used in the study. Bacterial identifications were performed by conventional and automated methods. The double disc synergy method was used to demonstrate the presence of ESBL. Molecular studies in all E. coli strains were performed by real-time PCR method.
Findings: 86 strains were studied, of which 83.72% were urine, 6.98% were wound, 4.65% were blood, and 2.33% were tracheal aspirate and sputum. 79.07% of these strains were ESBL-positive. 58.1% of the strains were female, whereas 41.9% were male patients, and the average age was 46.2. Out of 86 strains, 38.72% were ST131 positive, the H30 subclone was detected in 27.27% of them, and the H30-Rx subclone was detected in all of the H30 subclone positive strains. The presence of the ESBL resistance gene was detected at the rate of TEM 41.86%, SHV 37.21%, CTX-M 36.04%, and OXA 4.65%. Most commonly SHV gene (54.54%) was seen in ST131 clone-positive samples. Finally, while it was found that 48.83% of the strains formed biofilm by any method, biofilm formation was detected in 69.7% of the samples that were positive for the ST131 clone.
Result
Our study can reveal the dramatic prevalence of the ESBL-producing E. coli strains along with the high-risk ST131 clone, the dominance of the H30Rx subclone of this risky clone, as well as the importance of the influence of resistance mechanisms along with resistance and biofilm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Escherichia coli has an important place in a hospital and community-acquired infections. The most common reason for being a microorganism that is the causative agent of infection is that it is a typical flora element, as well as the virulence factors it holds and the resistance it has developed to the used antibiotics. E. coli ST131 usually exits in the digestive tract of mammals. However, its pathogenicity is generally defined as extra-intestinal pathogenic since E. coli (ExPEC) can progress into the bloodstream with the intermediation of the urinary tract [1]. This pathogen was first detected in 2008 and has appeared on three continents simultaneously [2]. E. coli ST131 belongs to group B2 as a phylogroup, and most clinical strains have the O25b: H4 serotype [3]. It is known that group B2 carries much more virulence factors [4].
In recent years, E. coli ST131 has been identified as a “high-risk pandemic clone” in the spread of antibiotic resistance worldwide and has become an important target for global surveillance studies [5, 6]. Conducted studies have revealed that the ST131 clone shows resistance to multiple drugs, mainly CTX-M and ESBL production, as well as quinolone resistance, thereby complicating the treatment response. Especially in our country, where fluoroquinolone group antibiotics are often used in empirical antibiotic therapy, we observe reports of increasingly high ESBL and antibiotic resistance rates in E. coli strains [7, 8]. Studies reveal that ESBL-producing E. coli strains are often associated with the CTX-M type enzyme [9, 10]. Furthermore, ESBL production is more frequently seen mainly for CTX-M in E. coli strains [11], whereas information about the surveillance of the ST131 clone is quite limited [12,13,14]. However, in recent studies, it has been reported that in addition to CTX-M enzymes, TEM and SHV-type ESBLs have also been observed in many countries [15].
Biofilm production of E. coli is an important virulence factor contributing to antimicrobial resistance to the action of antibiotics. Biofilm production by ST131 may protect bacteria from exposure to high levels of antibiotics and thus promote the development of resistance at low-level exposures. Few studies have investigated the biofilm production of E. coli ST131 isolates. These studies have reported high rates of biofilm production by ST131 strains [16,17,18]. They reported that this clone exhibits a specific O25b molecular subtype and produces biofilms, making it highly virulent, although it lacks the classic extraintestinal pathogenicity islands and afa/dra gene [16]. Similarly, the prevalence of biofilm production was significantly higher among ST131 isolates than among non-ST131 isolates, and a significant prevalence was also reported, being lowest in fecal isolates, higher in cystitis isolates, and highest in pyelonephritis isolates [17]. Some of these studies have reported contrasting findings with no specifically identified factor promoting ST131 biofilm. Studies in the literature suggest that Type 1 fimbriae expression is a critical determinant for biofilm formation by ST131 strains and that adhesion inhibition of Fim H has significant effects on biofilm formation [19]. This study aims to diagnose E. coli strains by phenotypic methods, compare their antimicrobial resistance profiles and biofilm formation characteristics with the detection or non-detection of clones of E. coli ST131 (O25pabBspe and trpA2) /H30 clone (fimH30)/subclone, and assess risk factors for ST131 carriage.
Materials and methods
Ethical approval
The study was approved in the Atatürk University Clinical Research Ethics Committee session dated 15/04/2021 with the decision no B.30.2.ATA.0.01.00/06/181. Ataturk University Scientific Research Projects received support with the project with no TKP-2021-9841.
Bacterial isolates and antibiotic susceptibilities
In our study, 86 E. coli isolates, 72 urinary and 14 non-urinary, isolated in the Microbiology Laboratory of Atatürk University Training and Research Hospital between April 2019 and December 2019 were used. Isolates resistant to at least three antibiotic groups (especially quinolones and cephalosporins) were selected for the study. Out of the 14 isolates, 6 were obtained from a wound, 4 from blood, 2 from tracheal aspirate, and 2 from sputum samples. In addition to conventional methods, BD Phoenix™ automatic AST system (Becton Dickinson, USA) was used to identify bacteria. Antibiotic susceptibility tests were performed using “European Committee on Antimicrobial Susceptibility Testing” (EUCAST) criteria with the BD Phoenix™ automatic AST system (Becton Dickinson, USA) or Kirby-Bauer disk diffusion method [20]. For this purpose, amikacin (AK) (30 µg), ampicillin-sulbactam (SAM) (10 µg), my ceftazidime (30 µg), ertapenem (ETP) (10 µg), gentamicin (CN) (10 µg), Cefixime (CFM) (5 µg) imipenem (IPM) (10 µg), meropenem (MEM) (10 µg), piperacillin-tazobactam I (TPZ) (110 µg), cefepime (FEB) (30 µg), ceftriaxone (CRO) (30 µg), cefotaxime (CTX) (30 µg), cefuroxime (CXM) (10 µg), ciprofloxacin (CIP) (5 µg), trimethoprim & sulfametokzasol (SXT) (25 µg) (Bioanalyse, Ankara, Turkey) antibiotic discs were used. The double disc synergy method was used to show the presence of ESBL [21]. The isolates were stored in a broth medium with 16% glucose at – 20 °C until molecular tests were performed.
Determination of biofilm forming capacities
The strains were removed from − 20 °C before being taken to the study. Nutrient agar (NA) was cultured and kept in the incubator at 37 ± 2 °C for 18 ± 2 h, and colonies obtained from fresh cultures were included in the study.
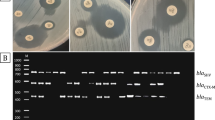
In the Congo red agar (CRA) method, Congo Red Agar was cultured for the bacteria produced in NA to form biofilm. Petris was incubated at 37 °C for 24 h. As shown in Fig. 1, after incubation, the formation of black colonies of bacteria in Petris was evaluated as positive, and the formation of red-pink colonies was evaluated as negative [22].
Congo red agar E. coli biofilm formation. R resistance, S susceptibility; *Amikak (EC) (30 µg), ampicillin-sulbactam (SAM) (10 µg), Ceftazidime (30 µg), Ertapenem (ETP) (10 µg), Gentamicin (CN) (10 µg), Cefixime (CFM) (5 µg) Imipenem (IPM) (10 µg), Meropenem (MEM) (10 µg), the piperasilli-tazobactam I (TPZ) (110 µg), Cefepime (FEB) (30 µg), Ceftriaxone (CRO) (30 µg), Cefotaxime (CTX) (30 µg), Cefuroxime(CXM) (10 µg), Ciprofloxacin (CIP) (5 µg), Trimethoprim and sulfametokzasol (SXT) (25 µg)
In the quantitative glass tube testing method, the single colony was produced on NA medium, cultured on Luria–Bertani (LB) broth medium, and left for incubation at 37 °C for 18–24 h at a speed of 160 rpm. After incubation, in an LB medium containing 1% glucose, it was inoculated to be at 0.5 McFarland standard turbidity (108 CFU/ml). After inoculation, from this medium, 100 µl was taken and transferred to an LB broth medium containing 1% glucose. After the transfer, the glass tubes were incubated at 37 °C at 160 rpm for 18–24 h. After incubation, the media were poured, and the tubes were washed with 2 ml sterile Phosphate Buffered Saline (PBS), and this process was repeated three times. Then, 2 ml of 1% crystal violet was transferred to the tubes and allowed to stain at room temperature for 20 min. After staining, the dye was removed by washing crystal violet three times with sterile PBS. The tubes were dried by turning them upside down on blotting paper. An 80:20 ethanol–acetic acid solution was added to the dried tubes and left for 10 min to dissolve the formed crystal violet ring. 1 ml of this dissolved dye was read in the spectrophotometer at OD540 nm, and biofilm formation (strong–weak) levels were detected. Biofilm formation in the tube and absorbance measurement were performed after staining with crystal violet [23].
In the quantitative microdilution plaque test method, after following the same steps that were applied in the quantitative glass tube test method to reach 0.5 McFarland standard turbidity setting, 20 µl of this medium and 180 µl of LB containing 1% glucose were placed in flat-bottomed microplate wells (1/100 dilution). Three wells were used for each bacterial and control strain. It was incubated at 37 °C at 160 rpm for 18–24 h. After incubation, the media were poured, and the microplates were washed gently with 200 µl sterile PBS, and this process was repeated three times. After then, 2 ml of 1% crystal violet was transferred to the microplates and allowed to stain at room temperature for 20 min. After staining, the dye was removed by washing crystal violet three times with 200 µl sterile PBS. The microplates were dried by turning them upside down on blotting paper. An 80:20 ethanol-acetic acid solution was added to the dried microplates and left for 10 min to dissolve the formed crystal violet ring. Biofilm formation (strong–weak) levels were detected by making microplates read at OD540nm on a multi-plate reader [24]. The level of forming biofilm of E. coli strains was calculated and evaluated using the following formula determined by Nasr et al. [25, 26].
DNA isolation
Genomic DNA was obtained by the boiling DNA method. The bacterial suspension produced during overnight incubation at 37 °C in 3 ml LB medium was centrifuged. The pellet was dissolved in 1000 ml of sterile water and centrifuged by vortexing again. After this process was repeated three times, it was vortexed in 300 ml of apyrogenic water and boiled for 10 min. Then it was centrifuged at 13,000 rpm for 10 min. Supernatants were used as molds in PCR tests [27].
Determination of ESBL genes by PCR
Using E. coli strain polymerase chain reaction (PCR), blaTEM, blaSHV, blaCTX-M1, and blaOXA genes were screened. The primers used for these genes are given in Table 1 [28]. PCR reaction components were prepared in a total volume of 25 µl (Table 2). PCR was performed under the reaction conditions specified in the references.
Determination of ST131 clone and subclones by molecular method
The first detection of O25pabBspe and trpA2 was examined to determine the presence of ST131 clones in isolated E. coli strains [29, 30]. First, a master mix was prepared to contain 10 × Reaction Buffer, dNTP mix (10 mm), MgCl2 (25 mM), (10 pmol) of each primer, (1 U) of Taq polymerase, 2 µl of mold DNA, 14.7 µl of distilled water components so that the total volume of the PCR is 25 µl.
The samples where the presence of O25pabBspe and trpA2 were observed were examined for the detection of the H30 subclone [20]. First, a master mix was prepared to contain 10× Reaction Buffer, dNTP mix (10 mm), MgCl2 (25 mM), (10 pmol) of each primer, (1 U) of Taq polymerase, 2 µl of mold DNA, 16.3 µl of distilled water components so that the total volume of the PCR is 25 µl. The samples where the presence of the H30 subclone was determined were examined for the presence of H30-Rx [29]. They were prepared the same way as the master mix quantities prepared for the H30 gene.
All PCR findings were analyzed on 1.5% agarose prepared with 5 µl Safe DNA gel and then examined under UV light.
Statistical analysis
In the statistical analysis of the data, the SPSS 15.0 program was used. The chi-square test was used to statistically evaluate the correlation between antibiotic resistance rates of isolates belonging and not belonging to the ST131 clone, and p < 0.05 was considered statistically significant.
Results
Out of 86 E. coli strains included in the study, 36 (41.9%) were isolated from male, and 50 (58.1%) were isolated from female patients. The ages of the patients were as follows respectively 10 of the patients were 0–5 years old, 11 of the patients were 6–15 years old, 4 of the patients were 16–29 years old, 27 of the patients were 30–59 years old; 34 of patients were were > 60 years old. 48 isolates (55.8%) were isolated from patients followed up in the Polyclinics, 30 (34.9%) isolates were isolated from patients followed up in departments, and 8 (9.3%) were from patients followed up in intensive care wards.
The antibiotic susceptibilities of E. coli strains were evaluated by disc diffusion test, and the presence of ESBL was evaluated by double disc synergy test (DST) in the same Petri. These results determined the resistance rates of E. coli strains developed against 15 different antibiotics (Fig. 2). 79.06% (n = 68) of the isolates were phenotypically determined as ESBL-positive.
Molecular results of ST131 clone and subclones in E. coli strains
Isolated E. coli strains were examined using multiplex PCR for the detection of O25pabBspe and trpA2 to determine the presence of the ST131 clone. It was determined that 38.37% [33] of 86 strains were ST131 (O25pabBspe and trpA2) positive, 65.11% [56] were only trpA2 positive, and 34.88% [30] were negative. 33 samples where O25pabBspe and trpA2 were present were examined for an H30 subclone, and an H30 subclone was detected in 27.27% [9] of them. In all E. coli strains where the H30 subclone was present, the H30-Rx subclone was also detected (Fig. 3).
ESBL was detected positive in 72.72% of the strains where ST131 was detected. It was determined that the isolates belonging to the ST131 clone had resistance to all examined antimicrobials such as ciprofloxacin (81.81%), cefuroxime (78.78%), cefepime (75.75%), ceftriaxone (72.72%), cefotaxime, TMP-SMZ, and I piperacillin-tazobactam (69.69%), cefixime (66.66%), ampicillin-sulbactam (63.63%) including carbapenems.
The results of ESBL resistance genes of E. coli strains
In all strains, TEM positivity was detected at a rate of 41.86%, SHV positivity was 37.21%, CTX-M positivity was 36.04%, and OXA positivity was 4.65%. None of the resistance genes studied were found in 32.55% of the isolates (Fig. 4).
While no resistance gene was found in 9.09% (3/33) of the samples with positive ST131 clones, at least one resistance gene was detected in 90.91% (30/33). In 54.54% (18/33) of the samples of positive ST131 clones, the SHV gene was detected. In 6.06% (2/33) OXA gene was detected. In 48.48% (16/33) CTX-M gene was detected, and in 51.51% (17/33) TEM gene was detected. When the correlation of ST131 clone and resistance genes was examined, a solid positive correlation was found with SHV (p < 0.005). It was determined that there was no statistically significant correlation between the ST131 clone and CTX-M, TEM, and OXA genes.
Results of biofilm forming capacities
Using the Congo Red Agar method, positivity was detected in 48.83% and 41.86% by glass tube test method, and all of them had mild severity, where positivity was detected by 45.34% using micro-dilution plaque method, 76.92% of them had moderate, 15.38% had weak, and 7.69% had moderate severity of positivity.
While 69.7% of the positive samples for the ST131 clone were detected to form biofilm by any method, 30.3% did not form any biofilm. When the correlation between the ST131 clone and biofilms was examined, a positive correlation with moderate severity was determined between the plate method and biofilm-positive strains and the ST131 clone (p < 0.05). It was determined that there was no statistically significant correlation between ST131 and biofilm tube, and CRA method and biofilm measurements (p > 0.05).
Discussion
By detecting the E. coli ST131 clone and comparing this clone with E. coli lineage, it is revealed that more virulence genes are carried and show multiple resistance to drugs and rapidly spread all over the world, and therefore constitutes a major problem for Global Public Health [31]. It is reported that the increase in the global spread of ESBLs is associated with better colonization of ST131 [32].
It is believed that the ST131 clone, contributes significantly to the spread of antibiotic resistance in E. coli strains. In our country, it is reported that resistance rates to antimicrobials among E. coli strains, especially quinolones, have been decelerating over the years [33, 34]. In our study, among ST131 isolates, ciprofloxacin, cefuroxime, cefepime, and cefotaxime resistance are high, and it was determined that they have resistance to antimicrobials, including carbapenems.
It was found that the E. coli ST131 clone was found in 12.5–30% of isolates isolated from clinical samples, almost in half of the isolates producing ESBL, and about 80% of the isolates resistant to fluoroquinolone [35]. In the study conducted by Yumuk et al. in 2008 for the first time in Turkey, one isolate in seventeen E. coli strains producing ESBL has been reported to be an ST131 clone [36]. In later studies, it has been reported that this rate is increasing more and more [12, 14, 37]. In our study, in which the isolates taken within one year in Erzurum were included, ESBL was found at a rate of 79.06%, and the presence of an ST131 clone (35.29%) was found at a relatively high level.
With the spread of the ST131 clone worldwide, it is known that it leads to various infections, including bacteremia and urinary tract infections of both community and hospital origin. A study by Çizmeci et al. in 2018 detected the presence of ST131 clones in 38% of 50 non-urinary isolates as a non-urinary infection agent [34]. Our study detected ESBL in 78.57% and ST131 in 42.86% of 14 non-urinary clinical isolates (6 wounds, 2 aspirates, 4 blood, and 2 sputum). Although within the scope of the study, more than half of the isolates (%55.8) are isolated from outpatients in Community-Acquired Infections in our region gives an idea about the prevalence of ST131 clone, it cannot be made clear the distinction between community or hospital-acquired infection can be seen as limitations of the study. However, the results constitute important preliminary data on the incidence of the ST131 clone in urine and non-urine E. coli infections in our region.
TEM and SHV derivatives from beta-lactamases have been determined as genes responsible for the spread of antibiotic resistance for E. coli ST131 in the mid-1990s [38]. Currently, the CTX-M type enzyme, isolated for the first time in Munich and significantly affecting cefotaxime, is responsible for antibiotic resistance [39]. In Our Country, studies on the analysis of resistance genes in E. coli ST 131 strains are limited. In a study by Can et al. among the 59 ESBL-positive isolates, 18 (31%) were found to belong to the ST131 clone [14]. In our study, the SHV gene was the highest in ST131 strains (54.54%).
Biofilm production by ST131 can protect bacteria from exposure to high levels of antibiotics and thus promote the development of resistance at low-level exposures. Very few studies investigate the biofilm production of E. coli ST131 strains. A study conducted in France found that 2 ST131 strains produced biofilm after 48 h [16]. In Australia, it was observed that 95.55% of 135 E. coli ST131 strains formed biofilm, and they reported that the ability of ST131 strains to form biofilm was significantly higher compared to non-ST131 strains [17]. A study conducted in China found that 70% of E. coli ST131 strains formed biofilm [18]. In our study, it was found that 69.7% of the samples that were positive for ST131 clone formed biofilm by any method, while 30.3% did not form biofilm.
Our study conducted in the hospital reveals that ESBL-producing E. coli strains in clinical isolates of the hospital indicate the dramatic prevalence of their isolate along with the high-risk ST131 clone, the dominance of the H30RX subclone of this risky clone, as well as the successful execution of resistance mechanisms together with the biofilm. Further research of this high-risk pandemic clone in our country is necessary to prevent its spread, to determine whether it has acquired new resistance genes, to determine effective empirical antibiotic treatment options, and to develop new strategies for controlling antibiotic resistance. Rapid detection of E. coli ST131 presence which has become a significant public health threat is very important for the implementation of effective targeted treatment, detection of ST131 reservoirs, risk factors, and understanding of resistance transmission pathways in terms of multidrug resistance and the global spread of this resistance.
Data availability
Data and materials are available from the authors upon request.
References
Manges AR, Geum HM, Guo A, Edens TJ, Fibke CD, Pitout JDD (2019) Global Extraintestinal pathogenic Escherichia coli (ExPEC) lineages Clin. Microbiol Rev 32:1–25
Nicolas-Chanoine M-H, Blanco J, Leflon-Guibout V, Demarty R, Alonso MP et al (2008) Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J Antimicrob Chemother 61:273–281
Dahbi G, Mora A, Mamani R, López C, Alonso MP et al (2014) Molecular epidemiology and virulence of Escherichia coli O16:H5-ST131: comparison with H30 and H30-Rx subclones of O25b:H4-ST131. Int J Med Microbiol 304:1247–1257
Russo TA, Johnson JR (2000) Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J Infect Dis 181:1753–1754. https://doi.org/10.1086/315418
Mathers AJ, Peirano G, Pitout JD (2015) Escherichia coli ST131: The quintessential example of an international multiresistant high risk clone. Adv Appl Microbiol 90:109–154
Rogers BA, Sidjabat HE, Paterson DL (2011) Escherichia coli O25b-ST131: a pandemic, multiresistant, community-associated strain. J Antimicrob Chemother 66:1–14
Aykan SB, Ciftci IH (2013) Antibiotic resistance patterns of Escherichia coli strains isolated from urine cultures in Turkey: a meta-analysis. Mikrobiyol Bul 47(4):603–618
Gur D, Hascelik G, Aydin ND et al (2009) Antimicrobial resistance in gram negative hospital isolates: results of the Turkish HITIT-2 Surveillance Study of 2007. J Chemother 21(4):383–389
Bayraktar B, Toksoy B, Bulut E (2010) Detection of bla(CTX-M) beta-lactamase genes in extended-spectrum betalactamase producing gram-negative bacteria. Mikrobiyol Bul 44(2):187–196
Çopur Çiçek A, Saral A, Duzgun AO et al (2013) Nationwide study of Escherichia coli producing extended-spectrum beta-lactamases TEM, SHV and CTX-M in Turkey. J Antibiot 66:647–650
Azap OK, Arslan H, Serefhanoğlu K et al (2010) Risk factors for extended-spectrum beta-lactamase positivity in uropathogenic Escherichia coli isolated from community-acquired urinary tract infections. Clin Microbiol Infect 16(2):147–151
Aktaş E, Otlu B, Erdemir D, Ekici H, Bulut E (2017) A first insight into Escherichia coli ST131 high-risk clone among extended-spectrum beta-lactamase-producing urine isolates in Istanbul with the use of matrix-assisted laser desorption/ionization time-of-flight mass-spectrometry and real-time PZR. Microb Drug Resist 23(8):1032–1036
Yumuk Z, Afacan G, Nicolas-Chanoine MH, Sotto A, Lavigne JP (2008) Turkey: a further country concerned by community-acquired Escherichia coli clone O25-ST131 producing CTX-M-15. J Antimicrob Chemother 62(2):284–288
Can F, Kurt-Azap Ö, İspir P et al (2016) The clinical impact of ST131 H30-Rx subclone in urinary tract infections due to multidrug resistant Escherichia coli. J Glob Antimicrob Resist 4:49–52
Livermore DM, Canton R, Gniadkowski M et al (2007) CTX-M: changing the face of ESBLs in Europe. J Antimicrob Chemother 59:165–174
Clermont O, Lavollay M, Vimont S, Deschamps C, Forestier C, Branger C, Denamur E, Arlet G (2008) The CTX-M-15-producing Escherichia coli diffusing clone belongs to a highly virulent B2 phylogenetic subgroup. J Antimicrob Chemother 61:1024–1028
Kudinha T, Johnson JR, Andrew SD, Kong F, Anderson P, Gilbert GL (2013) Escherichia coli sequence type 131 as a prominent cause of antibiotic resistance among urinary Escherichia coli isolates from reproductive-age women. J Clin Microbiol 51(10):3270–3276
Zhang S, Zhang Q, Huang J, Cao Y, Zhao Z, Li B (2021) Epidemic Potential of Escherichia coli O16:H41-ST131: compared with pandemic O25b:H30-ST131 lineage. Infect Drug Resist 8(14):2625–2632
Sarkar S, Vagenas D, Schembri MA, Totsika M (2016) Biofilm formation by multidrug resistant Escherichia coli ST131 is dependent on type 1 fimbriae and assay conditions. Pathogens Dis. https://doi.org/10.1093/femspd/ftw013
The European Committee on Antimicrobial Susceptibility Testing (2015) Breakpoint tables for interpretation of MICs and zone diameters. Version 5.0. http://www.eucast.org, Erişim tarihi. Accessed 6 Oct 2018
Clinical and Laboratory Standards Institute (2015) Performance Standards for Antimicrobial Susceptibility Testing; Twenty-fifth informational supplement. Clinical and Laboratory Standards Institute M100-S25
Freeman DJ, Falkiner FR, Keane CT (1989) New method for detecting slime production by coagulase negative staphylococci. J Clin Pathol 42(8):872–874
Öztürk FY, Darcan C, Kariptaş E (2022) The determination, monitoring, molecular mechanisms and formation of biofilm in E. coli. Braz J Microbiol 54(1):259–277
Christensen GD, Simpson WA, Younger JJ et al (1985) Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol 22:996–1006
Nasr AR, AbuShady MH, Hussein SH (2012) Biofilm formation and presence of icaAD gene in clinical isolates of staphylococci. Egypt J Med Hum Genet 13:269–274
Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic- VM (2000) A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods 40:175–179
Ausubel FM, Brient R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1995) Short protocols in molecular biology, 2nd edn. Wiley, New York, pp 2355–2363
Jegadeeshkumar D, Rajen KS, Nirmala P, Gopinath LR, Prakash B (2016) Determination of acquaintance between biofilm and extended spectrum β-lactamases producers from diarrheal stool isolates of Escherichia coli. Int J Curr Res Biosci Plantbiol 3(10):174–178
Russo TA, Johnson JR (2000) Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J Infect Dis 181(5):1753–1754
Colpan A, Johnston B, Porter S et al (2013) Escherichia coli sequence type 131 (ST131) subclone H30 as an emergent multidrug-resistant pathogen among US veterans. Clin Infect Dis 57(9):1256–1265
Merino I, Hernández-García M, Turrientes MC et al (2018) Emergence of ESBL-producing Escherichia coli ST131-C1-M27 clade colonizing patients in Europe. J Antimicrob Chemother 73(11):2973–2980
Shaik S et al (2017) Comparative genomic analysis of globally dominant ST131 clone with other epidemiologically successful extraintestinal pathogenic Escherichia coli (ExPEC) lineages. mBio 8(5):e01596-17
Er DK, Dündar D, Uzuner H (2022) Distribution of virulence determinants among Escherichia coli ST131 and its H30/H30-Rx subclones in Turkey. Acta Microbiol Immunol Hung 70(1):47–51
Çizmeci Z, Otlu B, Aktaş E, Ördekçi S, Açıkgöz Ö, Güleç N (2018) Investigation of high-risk ST131 clone in ESBL-producing Escherichia coli isolates isolated from urine and non-urinary clinical specimens with MALDI-TOF MS and real time PCR. Mikrobiyol Bull 52(1):13–22
Banerjee R, Johnson JR (2014) A new clone sweeps clean: the enigmatic emergence of Escherichia coli sequence type 131. Antimicrob Agents Chemother 58(9):4997–5004
Yumuk Z, Afacan G, Nicolas-Chanoine MH, Sotto A, Lavigne JP (2008) Turkey: a further country concerned by communityacquired Escherichia coli clone O25-ST131 producing CTX-M-15. J Antimicrob Chemother 62(2):284–288
Can F, Azap OK, Seref C, Ispir P, Arslan H, Ergonul O (2015) Emerging Escherichia coli O25b/ST131 clone predicts treatment failure in urinary tract infections. Clin Infect Dis 60(4):523–527
Blanc V, Leflon-Guibout V, Blanco J, Haenni M, Madec JY, Rafignon G et al (2014) Prevalence of day-care centre children (France) with faecal CTX-M-producing Escherichia coli comprising O25b:H4 and O16:H5 ST131 strains. J Antimicrob Chemother 69(5):1231–1237
Dahbi G, Mora A, Lopez C, Alonso MP, Mamani R, Marzoa J et al (2013) Emergence of new variants of ST131 clonal group among extraintestinal pathogenic Escherichia coli producing extended-spectrum beta-lactamases. Int J Antimicrob Agents 42(4):347–351
Pagani L, Dell’Amico E, Migliavacca R, D’Andrea MM, Giacobone E, Amicosante G, Romero E, Rossolini GM (2003) Multiple CTX-M-type extended-spectrum β-lactamases in nosocomial isolates of Enterobacteriaceae from a hospital in Northern Italy. J Clin Microbiol Sept 2003:4264–4269
Oliver A, Weigel LM, Rasheed JK, McGowan JE, Raney P, Tenover FC (2002) Mechanisms of decreased susceptibility to cefpodoxime in Escherichia coli. Antimicrob Agents Chemother 46(12):3829–3836
Hong F, Christina L, Barbro OL, Goran H, Emma L, Asa R (2004) Molecular epidemiological analysis of Escherichia coli isolates producing extended-spectrum beta-lactamases for identification of nosocomial outbreaks in Stockholm, Sweden. J Clin Microbiol 42:5917–5920
Funding
The authors did not receive any financial support.
Author information
Authors and Affiliations
Contributions
AT and YY performed the experiments and wrote the manuscript; SG, SY, and IB contributed significantly to the experiments and data analysis; AH contributed to the conception of the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Celebi, D., Aydın, E., Rakici, E. et al. Evaluation of presence of clone ST131 and biofilm formation in ESBL producing and non-producing Escherichia coli strains. Mol Biol Rep 50, 5949–5956 (2023). https://doi.org/10.1007/s11033-023-08532-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08532-z