Abstract
Background
Cyclosporine A (CsA)-induced cardiac interstitial fibrosis and cardiac hypertrophy are highly known phenomena; however, the basic mechanisms of CsA cardiotoxicity are unclear. The present study evaluated the role of the Transforming growth factor-beta (TGF-β)/Smad3/miR-29b signaling pathway and CaMKIIδ isoforms gene expression in cardiac remodeling under CsA exposure alone or combined with moderate exercise.
Methods
A total of 24 male Wistar rats were divided into control, cyclosporine (30 mg/kg BW), and cyclosporine-exercise groups.
Results
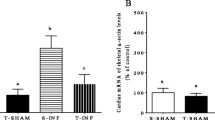
After 42 days of treatment, the findings revealed a significant decline in miR-29 and miR-30b-5p gene expression and an increase in gene expression of Smad3, calcium/calmodulin-dependent protein kinaseIIδ (CaMKIIδ) isoforms, Matrix Metalloproteinases (MMPs), protein expression of TGF-β, heart tissue protein carbonyl and oxidized LDL (Ox-LDL), and plasma LDL and cholesterol levels in the CsA-treated group compared to the control group. The CsA group presented greater histological heart changes such as fibrosis, necrosis, hemorrhage, infiltrated leukocyte, and left ventricular weight/heart weight than the control group. Moreover, combined moderate exercise and CsA relatively improved gene expression changes and histological alternations compared to the CsA group.
Conclusion
TGF-β-Smad3-miR-29 and CaMKIIδ isoforms may mainly contribute to the progression of heart fibrosis and hypertrophy due to CsA exposure, providing new insight into the pathogenesis and treatment of CsA-induced side effects on the heart tissue.





Similar content being viewed by others
Data Availability
The data that support the finding of this study are available from the corresponding author upon reasonable request.
Abbreviations
- TGF-β:
-
Transforming growth factor-beta
- CaMKII:
-
Calcium/calmodulin-dependent protein kinase II
- CsA:
-
Cyclosporine
- MMPs:
-
Matrix Metalloproteins
- Ox-LDL:
-
Oxidase-low-density lipoprotein
- ROS:
-
Reactive oxygen species
- PVDF:
-
Polyvinylidene Fluoride
- OS:
-
Oxidative Stress
- SD:
-
Smad-dependent
References
Bianchi R, Rodella L, Rezzani R (2003) Cyclosporine A up-regulates expression of matrix metalloproteinase 2 and vascular endothelial growth factor in rat heart. Int Immunopharmacol3(3):427–33. https://doi.org/10.1016/S1567-5769(03)00020-1
Rezzani R (2006) Exploring cyclosporine A-side effects and the protective role-played by antioxidants: the morphological and immunohistochemical studies. Histol Histopathol21(3):301–16. https://doi.org/10.14670/HH-21.301
Wan M, Xiao J, Liu J, Yang D, Wang Y, Liu J, Huang L, Liu F, Xiong G, Liao X (2023) Cyclosporine A induces hepatotoxicity in zebrafish larvae via upregulating oxidative stress. Toxicology &, Comparative Biochemistry and Physiology Part C. Pharmacology 266(109560
Selcoki Y, Uz E, Bayrak R, Sahin S, Kaya A, Uz B, Karanfil A, Ozkara A, Akcay A (2007) The protective effect of erdosteine against cyclosporine A-induced cardiotoxicity in rats. Toxicology 239(1–2):53–9. https://doi.org/10.1016/j.tox.2007.06.096
Tang J, Wang G, Liu Y, Fu Y, Chi J, Zhu Y, Zhao Y, Yin X (2011) Cyclosporin A induces cardiomyocyte injury through calcium-sensing receptor-mediated calcium overload. Pharmazie66(1):52–7
Rezzani R, Rodella LF, Fraschini F, Gasco MR, Demartini G, Musicanti C, Reiter RJ (2009) Melatonin delivery in solid lipid nanoparticles: prevention of cyclosporine A induced cardiac damage. J Pineal Res46(3):255 –61. https://doi.org/10.1111/j.1600-079X.2008.00651.x
Wu Q, Wang X, Nepovimova E, Wang Y, Yang H, Kuca K (2018) Mechanism of cyclosporine A nephrotoxicity: oxidative stress, autophagy, and signalings. Food and chemical toxicology 118:889–907
Ozkan G, Ulusoy S, Alkanat M, Orem A, Akcan B, Ersoz S, Yulug E, Kaynar K, Al S (2012) Antiapoptotic and antioxidant effects of GSPE in preventing cyclosporine A-induced cardiotoxicity. Ren Fail34(4):460–6. https://doi.org/10.3109/0886022X.2012.656563
Siwik DA, Colucci WS (2004) Regulation of matrix metalloproteinases by cytokines and reactive oxygen/nitrogen species in the myocardium. Heart Fail Rev9(1):43-51. https://doi.org/10.1023/B:HREV.0000011393.40674.13
Yesildag K, Gur C, Ileriturk M, Kandemir FM (2022) Evaluation of oxidative stress, inflammation, apoptosis, oxidative DNA damage and metalloproteinases in the lungs of rats treated with cadmium and carvacrol.Molecular Biology Reports1–11
Pohlers D, Brenmoehl J, Löffler I, Müller CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW, Wolf G (2009) TGF-β and fibrosis in different organs—molecular pathway imprints. Biochimica et Biophysica Acta (BBA)-Molecular basis of Disease. 1792(8):746–756
Ghosh S, Brauer PR (1996) Latent transforming growth factor-beta is present in the extracellular matrix of embryonic hearts in situ. Dev Dyn 205(2):126– – 34.10.1002/(SICI)1097 – 0177(199602)205:2 < 126::AID-AJA4 > 3.0.CO;2-K
Van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN (2008) Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proceedings of the National Academy of Sciences105(35):13027-32
He Y, Huang C, Lin X, Li J (2013) MicroRNA-29 family, a crucial therapeutic target for fibrosis diseases. Biochimie95(7):1355-9
Sassi Y, Avramopoulos P, Ramanujam D, Grüter L, Werfel S, Giosele S, Brunner A-D, Esfandyari D, Papadopoulou AS, De Strooper B (2017) Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating wnt signaling. Nature communications 8(1):1–11
Zhang T, Kohlhaas M, Backs J, Mishra S, Phillips W, Dybkova N, Chang S, Ling H, Bers DM, Maier LS (2007) CaMKIIδ isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses. J Biol Chem 282(48):35078–35087
Høydal MA, Wisløff U, Kemi OJ, Ellingsen Ø (2007) Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Prev Cardiol 14(6):753–760
Sadeghzadeh M, Shirpoor A, Naderi R, Kheradmand F, Gharalari FH, Samadi M, Khalaji N, Gharaaghaji R (2019) Long-term ethanol consumption promotes changes in beta-defensin isoform gene expression and induces structural changes and oxidative DNA damage to the epididymis of rats. Mol Reprod Dev86(6):624 –31. https://doi.org/10.1002/mrd.23138
Florio S, Ciarcia R, Crispino L, Pagnini U, Ruocco A, Kumar C, D’Andrilli G, Russo F (2003) Hydrocortisone has a protective effect on CyclosporinA-induced cardiotoxicity. J Cell Physiol195(1):21–6. https://doi.org/10.1002/jcp.10216
Davis PH, Dawson JD, Riley WA, Lauer RM (2001) Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: The Muscatine Study. Circulation104(23):2815 –9. https://doi.org/10.1161/hc4601.099486
Austin MA, King MC, Vranizan KM, Krauss RM (1990) Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation82(2):495-506. https://doi.org/10.1161/01.cir.82.2.495
Anber V, Griffin BA, McConnell M, Packard CJ, Shepherd J (1996) Influence of plasma lipid and LDL-subfraction profile on the interaction between low density lipoprotein with human arterial wall proteoglycans. Atherosclerosis124(2):261–71. https://doi.org/10.1016/0021-9150(96)05842-x
Kandasamy AD, Chow AK, Ali MA, Schulz R (2010) Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc Res85(3):413–23. https://doi.org/10.1093/cvr/cvp268
Rezzani R, Giugno L, Buffoli B, Bonomini F, Bianchi R (2005) The protective effect of caffeic acid phenethyl ester against cyclosporine A-induced cardiotoxicity in rats. Toxicology212(2–3):155–64. https://doi.org/10.1016/j.tox.2005.04.020
Chow AK, Cena J, Schulz R (2007) Acute actions and novel targets of matrix metalloproteinases in the heart and vasculature. Br J Pharmacol. 152(2):189-205. https://doi.org/10.1038/sj.bjp.0707344
Romanic AM, Harrison SM, Bao W, Burns-Kurtis CL, Pickering S, Gu J, Grau E, Mao J, Sathe GM, Ohlstein EH, Yue TL (2002) Myocardial protection from ischemia/reperfusion injury by targeted deletion of matrix metalloproteinase-9. Cardiovasc Res54(3):549 – 58. https://doi.org/10.1016/s0008-6363(02)00254-7
Tyagi SC, Matsubara L, Weber KT (1993) Direct extraction and estimation of collagenase(s) activity by zymography in microquantities of rat myocardium and uterus. Clin Biochem. 26(3):191–8. https://doi.org/10.1016/0009-9120(93)90025-2
Robert V, Besse S, Sabri A, Silvestre JS, Assayag P, Nguyen VT, Swynghedauw B, Delcayre C (1997) Differential regulation of matrix metalloproteinases associated with aging and hypertension in the rat heart. Lab Invest 76(5):729–738
Mahmoud NM, Elshazly SM, Rezq S (2022) Geraniol protects against cyclosporine A-induced renal injury in rats: Role of Wnt/beta-catenin and PPARgamma signaling pathways. Life Sci. 291:120259. https://doi.org/10.1016/j.lfs.2021.120259
Wu Q, Wang X, Nepovimova E, Wang Y, Yang H, Kuca K (2018) Mechanism of cyclosporine A nephrotoxicity: Oxidative stress, autophagy, and signalings. Food Chem Toxicol. 118:889-907. https://doi.org/10.1016/j.fct.2018.06.054
Gordon KJ, Blobe GC (2008) Role of transforming growth factor-β superfamily signaling pathways in human disease. Biochimica et Biophysica Acta (BBA)-Molecular basis of Disease. 1782(4):197–228
Lipke D, Newman P, Tofiq S, Guo H, Arcot S, Aziz S, Olson J, Soltis E (1997) Multiple polyamine regulatory pathways control compensatory cardiovascular hypertrophy in coarctation hypertension. Clin Experimental Hypertension. 19(3):269–295
Xiao Y, Ye J, Zhou Y, Huang J, Liu X, Huang B, Zhu L, Wu B, Zhang G, Cai Y (2018) Baicalin inhibits pressure overload-induced cardiac fibrosis through regulating AMPK/TGF-β/Smads signaling pathway. Archives of biochemistry and biophysics. 640:37–46
Inazaki K, Kanamaru Y, Kojima Y, Sueyoshi N, Okumura K, Kaneko K, Yamashiro Y, Ogawa H, Nakao A (2004) Smad3 deficiency attenuates renal fibrosis, inflammation,and apoptosis after unilateral ureteral obstruction. Kidney Int66(2):597-604. https://doi.org/10.1111/j.1523-1755.2004.00779.x
Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A (2003) Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest112(10):1486–94. https://doi.org/10.1172/JCI19270
Anderson ME, Brown JH, Bers DM (2011) CaMKII in myocardial hypertrophy and heart failure. Journal of molecular and cellular cardiology51(4):468 – 73
Maier L (2005) CaMKIIdelta overexpression in hypertrophy and heart failure: cellular consequences for excitation-contraction coupling. Braz J Med Biol Res 38(9):1293–1302
Zhang T, Miyamoto S, Brown JH (2004) Cardiomyocyte calcium and calcium/calmodulin-dependent protein kinase II: friends or foes? Recent progress in hormone research. 59(1):141–168
Zhang L, Jia X (2019) Down-regulation of miR-30b-5p protects cardiomyocytes against hypoxia-induced injury by targeting Aven. Cellular & molecular biology letters. 24(1):1–10
He J, Jiang S, Li F-l, Zhao X-j, Chu E-f, Sun M-n, Chen M-z, Li H (2013) MicroRNA-30b-5p is involved in the regulation of cardiac hypertrophy by targeting CaMKIIδ. Journal of investigative medicine61(3):604 – 12
Palomeque J, Rueda OV, Sapia L, Valverde CA, Salas M, Petroff MV, Mattiazzi A (2009) Angiotensin II–induced oxidative stress resets the Ca2 + dependence of Ca2+–calmodulin protein kinase II and promotes a death pathway conserved across different species. Circul Res 105(12):1204–1212
Heshmati E, Shirpoor A, Kheradmand F, Alizadeh M, Gharalari FH (2018) Chronic ethanol increases calcium/calmodulin-dependent protein kinaseIIdelta gene expression and decreases monoamine oxidase amount in rat heart muscles: Rescue effect of Zingiber officinale (ginger) extract. Anatol J Cardiol19(1):19-26. https://doi.org/10.14744/AnatolJCardiol.2017.8079
An N, Chen Y, Xing Y, Wu H, Gao X, Chen H, Song K, Li Y, Li X, Yang F (2020) Cardiac CaMKIIδ and Wenxin Keli prevents Ang II-Induced Cardiomyocyte Hypertrophy by modulating CnA-NFATc4 and Inflammatory Signaling Pathways in H9c2 cells.Evidence-Based Complementary and AlternativeMedicine2020(1–17
Hung W, Fang S-H, Wu C-L, Ko M-H, Liu T-H, Chang C-K (2013) Regular endurance exercise prevents cyclosporine A-induced oxidative stress in mouse skeletal muscles. Science & sports28(5):295-9
Gliemann L, Nyberg M, Hellsten Y (2016) Effects of exercise training and resveratrol on vascular health in aging. Free Radical Biology and Medicine98(165 – 76
Bayod S, Del Valle J, Lalanza J, Sanchez-Roige S, de Luxan-Delgado B, Coto-Montes A, Canudas A, Camins A, Escorihuela RM, Pallas M (2012) Long-term physical exercise induces changes in sirtuin 1 pathway and oxidative parameters in adult rat tissues. Experimental gerontology47(12):925 – 35
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Khatereh nourmohammadi: Conceptualization, Methodology, Software abolfazl bayrami.: Data curation, Writing- Original draft preparation. Roya naderi: Visualization, Investigation. Alireza shirpoor: Supervision. All authors read and approved the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
the Local Ethics Committee on Animal Experiments at Mohaghegh Ardabili University in Iran confirmed this study (IR.UMA.REC.1400.032).
Conflict of interest
The authors declare there are no competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.








Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nourmohammadi, K., Bayrami, A., Naderi, R. et al. Cyclosporine A induces cardiac remodeling through TGF-β/Smad3/miR-29 signaling pathway and alters gene expression of miR-30b-5p/CaMKIIδ isoforms pathways: alleviating effects of moderate exercise. Mol Biol Rep 50, 5859–5870 (2023). https://doi.org/10.1007/s11033-023-08506-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08506-1