Abstract
Background
MiRNAs can affect the radiosensitization of head and neck squamous cell carcinoma (HNSCC). We aimed to analyze the function of miR-125 family members in HNSCC using The Cancer Genome Atlas (TCGA) and determine their effect on radiation in laryngeal squamous cell cancer (LSCC).
Methods
First, we systematically analyzed the role of the miR-125 family in HNSCC using the TCGA database and found that miR-125a-5p is associated with radiotherapy. We then performed comprehensive enrichment analysis of miR-125a-5p and predicted target genes. Then, we performed transfection, cell proliferation assays, reverse transcription polymerase chain reaction, apoptosis assays, micronucleus tests, and western blotting on hep-2 cells selected with puromycin.
Results
MiR-125 family members exhibited significantly different expression in HNSCC. They were significantly associated with tumor–node–metastasis staging, clinical stages, and histological grades. Radiation therapy had a statistically effect on miR-125 family members, except miR-125a-3p. Moreover, miR-125a-5p was related to overall survival in LSCC. Thus, we predicted 110 target genes and seven hub genes of miR-125a-5p. The proliferation rate of cells transfected with lentivirus vector expressing miR-125a-5p was significantly reduced compared to the other groups. The radiation effect was enhanced in cells transfected with miR-125a-5p. The ratio of apoptotic cells transfected and exposed to X-rays (10 Gy) was distinctly higher than that of the Ad-control group. Western blotting analysis revealed that miR-125a-5p upregulated the apoptotic regulators P53 and rH2AX. Thus, miR-125a-5p may increase radiosensitivity in LSCC via upregulation of pro-apoptotic genes.
Conclusions
MiR-125 family members could be prognostic biomarkers of HNSCC and improve HNSCC sensitivity to radiotherapy by activating P53. Upregulating miR-125a-5p via lentivirus vectors may be a novel strategy to strengthen the effect of radiotherapy on LSCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is one of the top ten most common malignancies in the world, accounting for almost 90% of head and neck malignant tumors [1]. HNSCC may occur in the oral cavity, nasal cavity, nasopharynx, larynx, and thyroid [2]. Laryngeal squamous cell cancer (LSCC) is the most common form of HNSCC. Despite significant progress in the multimodal treatment of HNSCC, which includes radiotherapy, chemotherapy, and surgery, the survival rate of patients with HNSCC is still low [3]. As radiotherapy is a key nonsurgical approach for HNSCC, the sensitivity of HNSCC to radiotherapy should be urgently improved.
MiRNAs, which are single-stranded RNAs of 18–25 nucleotides, can adjust gene expression in various ways via mRNA degradation or transcription inhibition [4]. Several relevant miRNAs have been proven to influence the proliferation, invasion, apoptosis, chemosensitivity, and radiosensitization of HNSCC. Among the miRNAs associated with tumor progression the miR-125 family is composed of five paralogs (miR-125a-3p, miR-125a-5p, miR-125b-1-3p, miR-125b-2-3p, and miR-125b-5p) that have an important function in multiple cancers, and exhibit the same seed sequence and similar effects in tumors. For example, the miR-125 family plays a role in the survival of patients with non-small cell lung cancer (NSCLC) [5]. MiR-125 family members can also reassess the prognostic values in the patients of gastric cancer and have been implicated in leukemia and lymphoma [6, 7]. However, the functional relationship between miR-125 family and HNSCC has not been clarified.
In this study, we probed the correlation between the miR-125 family members and HNSCC and detected effect of miR-125a-5p on radiotherapy in patients with LSCC. Through in-depth analysis, it was confirmed that miR-125a-5p was very important in the treatment of LSCC and could improve the effect of radiotherapy. Therefore, this study provides a modern radiotherapeutic approach for laryngeal cancer.
Materials and methods
The Cancer Genome Atlas (TCGA) analysis
MiRNA-sequencing and clinical data were collected from the TCGA dataset (https://portal.gdc.cancer.gov/). Level 3 BCGSC, IlluminaHiSeq, and miRNA-Seq data were adopted for pan-cancer analysis, including each tumor subtype. In addition, we downloaded TCGA miRNA-Seq data of HNSCC and all relevant clinical information. All paired samples were compared using the Wilcoxon signed-rank test. Statistical analyzes were performed with R software (version 3.6.3), and the ggplot2 package (version 2.2.1) was used to visualize the results. The relationships between the expression of miR-125 family members and pathological tumor–node–metastasis (TNM) stage, cancer stage, tumor grade, and radiation therapy in HNSCC were then analyzed. One-way analysis of variance (ANOVA) was used to compare TNM classification and cancer stages I–IV. The Wilcoxon rank sum test was used to analyze the effect of radiotherapy, and the Kruskal–Wallis test was used to compare tumor grade. MiRNA expression in LSCC and relevant clinical information were downloaded from the TCGA database. Overall survival (OS) was analyzed using the Log-rank (Mantel–Cox) test.
Functional enrichment analysis
Target genes were predicted using TarBase v.8 (http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=tarbasev8%2Findex), StarBase (http://starbase.sysu.edu.cn/), and miRWalk (http://mirwalk.umm.uni-heidelberg.de/). Venn diagrams were created to determine the intersection of the three datasets using the R package VennDiagram. Gene ontology (GO) annotation and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of the target genes were performed using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) (https://david.ncifcrf.gov/). Protein–protein interaction (PPI) networks of the target genes were retrieved from the STRING database (version 11.5) (https://string-db.org/cgi/input.pl). Cytoscape (version 3.8.0) was used to visualize the network, and the plug-in MCODE (version 1.5.1) was used to identify the most significant hub genes [8].
Cell culture
Hep-2 cell lines were purchased from the cell resources center of Hunan Fenghui Biotechnology Co., Ltd (Hunnan, China). Before the experiment, the hep-2 cells were conducted STR identification, which proved that the cells were not contaminated. The cells were then cultivated at 37 °C under 5% CO2 in sterile T25 culture flasks in RPMI 1640 medium. Cells were subcultured after reaching approximately 80% confluence.
Lentivirus vector preparation and generation of stable cell lines
The lentivirus vector miR-125a-5p was prepared as described by Lyu, H. et al. [9]. MiR-125a-5p kits were obtained from Sangon Biotech Engineering Co., Ltd. (Shanghai, China). Total RNA was extracted according to the manufacturer’s instructions. Third-generation hep-2 cells were seeded at 3 × 104 cells/mL in 6-well plates and transfected with lentiviral plasmid particles (MOI = 10) carrying miR-125a-5p or an empty vector. The stably transfected cells were selected with puromycin (1 µg/mL). The hep-2 cells were classified into three groups: the parental group, which was not infected and was cultured under initial conditions of observation; Ad-miR-125a-5p group, which was transfected with lentiviral particles containing miR-125a-5p; and Ad-control group, which was transfected with lentiviral particles containing negative control miRNA that did not interfere with any genes.
Detection of transfection efficiency using RT-PCR
According to the instructions of the Rneasy Plus Mini Kit (74134 and 74136, Qiagen, Germany), total RNA was extracted from the Ad-control group and Ad-miR-125a-5p group using Trizol solution and reverse-transcribed into cDNA. RT-PCR was performed using Faststart Universal SYBR Green Master (Roche) with the PCR detection system QuantStudio®3. The sequences of the primers used were as follows: U6 forward sequence: 5′-CTCGCTTCGGCAGCACA-3′, reverse sequence: 5′-AACGCTTCACGAATTTGCGT-3′; MiR-125-a-5p primers: GTCGTATCCAGTGCAG GGTCCGAGGTATTCGCACTGGATACGACTCACAG, MiR-125-a-5p forward sequence: 5′-CGCCGTCCCTGAGACCCTTTAAC-3′, MiR-125a-5p reverse sequence: 5′-ATCCAGTGCAGGGTCCGAGG-3′ (both from Sangon Biotech Engineering Co., Ltd. Shanghai, China). The relative expression of miR-125a-5p was calculated by the 2−ΔΔCt method.
MTT proliferation assay
The three groups of hep-2 cells were inoculated and cultured for 96 h and then harvested for the MTT assay. The hep-2 cells were added to the 96-well plates, and 100 µL of cell medium was added for routine culture in an incubator with 5% CO2. After culturing for 24, 48, 72, and 96 h, we added 20 µL of MTT (5 mg/mL in phosphate-buffered saline) into each well and cultured the cells for 4 h. Once the culture was terminated and the supernatant was carefully absorbed, we added 150 µL of dimethyl sulfoxide into each well and oscillated the mixture or 10 min to ensure that all crystals were fully dissolved. Absorbance values were measured on the iMark microplate reader (BioRad) at 490 nm and the results were recorded. GraphPad Prism 8.0 software was used to analyze the results of the paired t-test.
Colony formation assay
The hep-2 cells were plated in Petri dishes at 500, 1,00, 2000, 4000, 8000, and 16,000 cells/well and radiated via X-ray at 0, 2, 4, 6, 8, and 10 Gy for 2–4 weeks at 37 °C. The culture was terminated when colonies were macroscopically visible in the culture dish. The surviving cells were fixed with methanol for 15 min, stained with Giemsa for 20 min, and then counted as colonies. The colony formation rate of the 0-Gy group was used as the control to calculate the cell survival fraction (SF) under different irradiation doses. The plating efficiency (PE) and SF were calculated as follows: PE = number of clones/number of cells inoculated × 100%; SF = number of clones in the experimental group/ (number of inoculated cells × PE). GraphPad Prism 4.0 software was used to analyze the data, and the SF was obtained from the multitarget/single-hit model: SF = 1 − (1 − e−D/D0)N.
Apoptosis and flow cytometry analyses
The hep-2 cells were planted in 60-mm dishes, and the Ad-control group and Ad-miR-125a-5p group were exposed to X-ray doses of 10 Gy. After 48 h, apoptosis was surveying through the Annexin V apoptosis detection kit. Finally, the ratio of apoptosis was analyzed by flow cytometry (BD FACS Calibur, USA) and Cell Quest Software (Modfit LT for Mac 3.0). The differences were determined using SPSS statistical software (version 23, IBM, SPSS), and the results were analyzed by paired t-test.
Antibodies and western blotting
The three groups of hep-2 cells were exposed to different X-ray doses after 24 h. Total protein was extracted with RIPA buffer solution (Solarbio PMSF, Beijing, China), and the protein concentration was measured by bicinchoninic acid assay. SDS-PAGE Protein Loading Buffer was added to the protein samples, and membrane transfer (nitrocellulose membrane, microporous) were then conducted. After washing the membrane with TBS-T, the membrane was transferred to 10% bovine serum albumin and sealed in TBS-T buffer. After shaking at room temperature for 2 h, the membrane was closed and placed in a pouch containing the primary antibody diluent; the specific primary antibodies were rH2AX (microporous, 1:1,000), P53 (Abcam,1:1,000), and GAPDH (Zsbio, 1:5,000). After incubation with primary antibodies, the membrane was incubated in TBS-T and washed three times by Western wash, the secondary antibody diluent was added (Zsbio, 1:5,000). After rewashing the TBS-T membrane, a ChemiDoc-XRS system was used for analysis and a western blotting experiment was performed. Protein imaging was performed via a gel imaging system. With GAPDH as a reference, Gelpro32 was used to quantitatively analyze the grey values of protein bands. SPSS statistical software was used to identify the difference.
Results
Pan-cancer analysis of miR-125 family members
In the pan-cancer analysis, miRNA expression of miR-125 family members in 17 types of tumors was compared with that in normal tissues (Fig. 1). Paired t-test analysis indicated a significant difference in the expression of miR-125 family members in HNSCC tumor tissue (Fig. 1). MiR-125a-3p expression was higher in tumor tissue than in normal tissues, and the difference between the two groups was 0.268 (0.004–0.532), with statistical significance (t = 2.050, P = 0.047). The expression of other members of the miR-125 family was lower in tumor tissue than in normal tissues.
Expression of miR-125 family members in 17 different types of cancer (TCGA database). Significant changes in the expression of miR-125 family members between HNSCC tissue and normal tissue. The colour is correlated with the expression levels of miR-125 family members, with blue representing high expression and red representing low expression. *p < 0.05 and **p < 0.01, differences were compared using the Wilcoxon signed-rank test
Association of different miR-125 family members with clinicopathological parameters in HNSCC
Regarding clinical cancer stages (Fig. 2A), the Bonferroni multiple hypothesis test (Tukey’s honestly significant difference (HSD) post-hoc test) revealed that miR-125a-3p and miR-125a-5p showed no statistical difference between tumor and normal tissues at each stage. Conversely, miR-125b-1-3p, miR-125b-2-3p, and miR-125b-5p expression exhibited statistically significant differences at stages I–IV (P < 0.05).
Relationship between miR-125 expression in different clinical stages and grades of patients and TNM stages with HNSCC. A As the tumor stage increased, the expression of miR-125b-1-3p, miR-125b-2-3p, and miR-125b-5p in tumor tissue was lower than that in normal tissue. B The same results were observed in grades G1–G3. C, E MiR-125b-1-3p, miR-125b-2-3p, and miR-125b-5p expression was significantly correlated with clinical T and M stages. D The expression of all members of the miR-125 family, except miR-125a-5p, was significantly correlated with N1 and N2 stages. (F) A significant relationship was observed between the expression levels of miR-125a-5p, miR-125b-1-3p, miR-125b-2-3p, and miR-125b-5p and radiation therapy. *p < 0.05, **p < 0.01, and ***p < 0.001
Regarding the early histological grades (Fig. 2B), the Bonferroni multiple hypothesis test (Tukey's HSD post hoc test) revealed that miR-125a-3p expression was higher at stage G3 than at stage G1. The results of miR-125b-1-3p, miR-125b-2-3p, and miR-125b-5p expression according to the Bonferroni correction significance level of multiple hypothesis testing (Tukey's HSD post hoc test) revealed a significantly lower average expression level at stages G1, G2, and G3 in tumor tissue than in normal tissue.
Diagnostic value of miR-125 family in the TNM stage of HNSCC
For the TNM stage of HNSCC, the difference in miR-125a-5p and miR-125a-3p expression between normal and tumor tissues was not statistically significant at the T stage (Fig. 2C). However, miR-125b-1-3p, miR-125b-2-3p, and miR-125b-5p expression was significantly decreased in tumor tissues at T1, T2, T3, and T4 compared to that in normal tissues (Fig. 2C). In N staging, the Bonferroni method was used to correct the significance level of the multiple hypothesis test (Game–Howell post hoc test), which revealed that average miR-125 expression levels were lower in tumor tissues than in normal tissue at N0, N1, and N2 (Fig. 2D). No other comparisons were statistically significant. In M staging, miR-125b-1-3p, miR-125b-2-3p, and miR-125b-5p were significantly downregulated in tumor tissue at M1 and M2 (Fig. 2E). Furthermore, the expression of all miR-125 family members, except miR-125a-3p, was significantly lower in the radiation therapy group than in the normal tissue group (Fig. 2F).
Prognostic value of miR-125a family members in LSCC patients
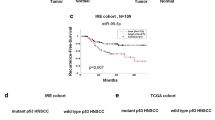
Survival analysis of miR-125a family members in LSCC revealed that only the expression levels of miR-125a-5p were correlated with OS (P = 0.026, Fig. 3A–E); therefore, we performed further analysis on this mRNA. The intersection of the target genes of miR-125a-5p determined using public databases, such as Starbase, miRWalk, and Tarbase, revealed a total of 110 target genes (Fig. 3F).
Prognostic value of the expression of miR-125 family members in LSCC. Generally, B upregulation of miR-125a-5p was significantly related to a favorable OS of patients with LSCC. However, (A, C–E) other miR-125 family members exhibited no correlation with prognosis in LSCC. F The target genes of miR-125a-5p were predicted using public databases (Starbase, miRWalk, and Tarbase), and 110 common genes were generated for further analysis
Functional enrichment analysis of miR-125a-5p
To further analyze the function of the target genes of miR-125a-5p, we performed GO and KEGG pathway enrichment analyses (Fig. 4). The molecular functions included protein binding, protein homodimerization activity, and enzyme binding (Fig. 4B). The biological processes included transcription, negative regulation of the RNA polymerase II promoter, and viral processes (Fig. 4A). The cellular components included cytosol, membrane, and nucleoplasm (Fig. 4C). KEGG pathway analysis further underscored the signaling pathways of cells, including the MAPK signaling pathway, viral carcinogenesis, proteoglycans in cancer, hepatitis C, the VEGF signaling pathway, and apoptosis (Fig. 4D). A PPI network was generated by the STRING database, and visualization of the network was performed by Cytoscape software (Fig. 4E). The following seven hub genes were identified by MCODE, an application of Cytoscape software: P53, VEGFA, FGFR1, STAT3, CFLAR, TNFRSF1B, and TNFRSF10B (Fig. 4F).
MiR-125a-5p enhances cellular radiosensitivity and X-ray-induced apoptosis
We proved that the expression level of miR-125a-5p in hep-2 cells transfected with the lentivirus vector was significantly higher than that in the un-transfected parental group (Fig. 5A). Next, we demonstrated the function of miR-125a-5p in cell proliferation. Initially, there was no obvious difference in the cell growth rate between Ad-control and Ad-miR-125a-5p (Fig. 5B). However, over time, the proliferation rate of cells transfected with the lentivirus vector of miR-125a-5p markedly decreased compared with that of the parental cells and cells of the negative control group transfected with lentivirus. These results revealed that the proliferation of Hep-2 cells was controlled by miR-125a-5p.
MiR-125a-5p may inhibit hep-2 cell proliferation and heighten the effect of radiotherapy and X-ray-induced apoptosis. A Reverse transcription polymerase chain reaction analysis was used to measure the total RNA of miR-125a-5p in the Ad-miR-125a-5p or Ad-control group. B The MTT assay confirmed that the relative number of cells was markedly reduced after transfection with miR-125a-5p. C The hep-2 cells irradiated with different X-ray doses, differences were significant between the parental cells and Ad-control or Ad-miR-125a-5p cells (P < 0.05). Ad-control (E, G) and (F, H) Ad-miR-125a-5p cells subjected to (G, H) 10 Gy of X-ray radiation or (E, F) untreated and harvested after 24 h. D Flow cytometry was used to measure the ratio of apoptotic cells, significant difference was observed between the Ad-control and Ad-miR-125a-5p groups (P < 0.05). *p < 0.05, **p < 0.01, and ***p < 0.001
Next, colony formation assay demonstrated the effect of miR-125a-5p on radiotherapy. The SF decreased significantly, and the decline in Ad-miR-125a-5p expression with increasing radiation dose was more pronounced than that in the other two groups (Fig. 5C). We also detected the effect of X-ray exposure on apoptosis using flow cytometry. The rate of apoptotic cells was substantially increased (from 1.10 to 12.46%) after transfection with miR-125a-5p (Fig. 5E, F). The X-ray effects were further enhanced in cells transfected with miR-125a-5p mimics (Fig. 5F). Additionally, exposure to 10 Gy of X-ray radiation increased the apoptosis rate (from 1.10 to 6.30%) (Fig. 5E, G). Under the same radiotherapy conditions, the rate of apoptosis increased (from 6.30% to 18.15%) in mock-transfected cells compared with that in cells transfected with miR-125a-5p mimics Fig. 5G, H). These results confirmed that miR-125a-5p substantially increased cell sensitivity to radiotherapy.
MiR-125a-5p enhances the expression of apoptosis genes
Next, western blotting analysis confirmed that the expression of P53 and γH2AX in all groups was increased after radiation treatment (P < 0.05) (Fig. 6A). After subjecting to X-ray radiation at 10 Gy, the expression of P53 was markedly higher in the Ad-miR-125a-5p group than in the Ad-control group (Fig. 6B). Moreover, the expression of γH2AX in the Ad-miR-125a-5p group was considerably higher than that in the Ad-control group (Fig. 6C). These results confirmed that miR-125a-5p can induce chromosomal damage by promoting p53 activity. Thus, overexpression of miR-125a-5p may increase radiosensitivity by promoting the expression of p53.
Discussion
HNSCC development is a complicated process involving multifactor participation, and miRNA plays a critical role in the regulation of HNSCC. Radiotherapy is a crucial method for the treatment of HNSCC, including LSCC. Therefore, the discovery of new and effective radiotherapy related genes is the key to the successful treatment of LSCC, and can also provide a new approach for the treatment.
The miR-125 family is related to the development and treatment of cancer and may even impact the survival outcomes of patients. MiR-125a-3p affects tamoxifen resistance in breast cancer patients. [10]. Another study indicated that miR-125b-1-3p had anticancer effects and was a diagnostic marker for pancreatic cancer [11]. MiR-125b-2-3p inhibits the TNM-based progression of HNSCC [12]. MiR-125b-5p may also promote the progression of LSCC and is an essential drug target [13, 14].
In this study, we first systematically studied the miR-125a family using the TCGA database and then performed pan-cancer analysis. MiR-125 expression differences were statistically significant in many cancers including breast invasive cancinoma, colon adenocarcinoma, liver cancer and so on. All members of the miR-125 family exhibited significantly different expression levels in HNSCC. We then conducted a comprehensive and systematic analysis of the clinical parameters related to HNSCC, including the TNM stage, clinical stage, histologic grade, and effect of radiation therapy. Furthermore, radiation therapy had a statistically significant effect on the expression of all members of the miR-125 family, except miR-125a-3p. Subsequently, we conducted a survival analysis of patients with LSCC and found that miR-125a-5p was related to the survival time of LSCC. Therefore, we further analyzed the correlation between miR-125a-5p and LSCC sensitivity to radiotherapy.
MiR-125a-5p was significantly differentially expressed between tumor tissues and normal tissues and was related to the occurrence, growth, and invasion of LSCC, so it may represent a new biomarker for biotherapy [15]. Our research corroborates these conclusions and reveals the mechanisms of actionof miR-125a-5p in laryngeal carcinoma for the first time. Our results demonstrated that the growth and invasion ability of hep-2 cells were decreased, and the radiosensitivity was increased through the overexpression of miR-125a-5p; therefore, miR-125a-5p may function as an antineoplastic gene in hep-2 cells. The GO terms confirmed that differentially expressed target genes were related to signal transduction by the p53 class mediator, resulting in cell cycle arrest and DNA damage response (DDR). In the KEGG analysis, apoptosis was significantly enriched. The results of the KEGG enrichment analysis were consistent with those of the GO enrichment analysis. Seven hub genes were obtained by Cytoscape. Among them, p53 is very highly correlated with human tumors. P53 expression not only promotes apoptosis, but also inhibits the growth of tumors. When cancer develops further, p53 gene mutations are inactivated and apoptosis is inhibited. After radiotherapy, p53-mediated apoptosis is initiated [16].
Radiotherapy can induce cell apoptosis and chromosomal damage. Typical biochemical changes during radiation-induced apoptosis include single-chain breaks, endonuclease activation, and DNA double-strand breaks (DSBs), which play an important role in radiotherapy [17]. During the repair of DNA DSBs, the DNA damage sensors H2AX, RNF8, RAD50, ATM, ATR, and XRCC4, as well as many other molecules, play important roles in the DDR process [17]. The DNA repair mechanism of tumor cells is one of the main reasons for tumor radiation resistance, which is mainly related to repair proteins.
Increasing evidence supports the hypothesis that miRNAs are involved in the regulation of DNA repair proteins, thereby affecting the sensitivity of tumor cells to radiotherapy. For example, MiR-146a-5p can activate the DNA repair pathway and increase radiosensitivity in hepatocellular carcinoma [18]. Moreover, MiR-3607 could regulate the DNA damage repair pathway through targeting DDI2 and decrease the tumorigenesis in colorectal cancer [19].
In this study, miR-125a-5p reduced the repair ability of DNA damage by regulating the expression of rH2AX when DNA double-strand damage occurred. H2AX is a histone located in the nucleus of cells and is phosphorylated to form rH2AX when DNA strand damage is followed by DSB [17]. rH2AX level decreases with DNA repair and is then dephosphorylated when repair is complete. rH2AX is therefore, an index of reactive DNA repair. In this study, we confirmed that, in cells overexpressing miR-125a-5p, rH2AX level was significantly increased, DNA repair was inhibited, DSBs were promoted, cell apoptosis was inhibited, and tumor growth was inhibited under X-ray radiation at 10 Gy, which enhanced tumor sensitivity to radiotherapy. During tumor radiotherapy, p53 plays an important role in apoptosis. By activating or inhibiting a series of genes, wild-type p53 can promote tumor cell apoptosis and enhance the sensitivity of tumors to radiotherapy by inhibiting the tumor cell cycle and the repair of radiation damage in tumor cells [19]. Thus, the wild-type p53 gene plays an important role in the control of cell cycle arrest, DNA repair, and apoptosis. However, after mutation, the p53 gene loses its normal function, and the apoptosis process of tumors is inhibited after radiotherapy, resulting in resistance to radiotherapy [19]. Many studies have shown that wild-type p53 can increase the radiosensitivity of HNSCC [17].
The experimental results showed that miR-125a-5p could improve the sensitivity of tumor cells to radiotherapy by upregulating p53 and enhancing apoptosis in lung cancer. Similarly, a previous study found that miR-125a-5p contributes to p53 activation [20]. Following radiation therapy-induced DSB, two repair pathways are activated: homologous recombination and nonhomologous end joining (NHEJ), the most important pathway is NHEJ. However, the p53 gene can inhibit the NHEJ repair system, so that the double-stranded broken DNA cannot be repaired and tumor cells can undergo apoptosis. Control of the cellular response to radiotherapy by inhibiting DDR has been a focus of current radiotherapy research. In this study, we found that apoptosis of hep-2 cells was significantly increased by radiation treatment after upregulation of miR-125a-5p, indicating that miR-125a-5p has a radiotherapy sensitization effect. P53 activation plays an important role in DNA damage transduction and a pivotal role in processing "subnodes" in the DNA damage signal network. Our study proved that miR-125a-5p can upregulate rH2AX and p53 and inhibit DNA damage repair. Under the condition of radiotherapy, DNA damage and miR-125a-5p can induce DSBs and rH2AX phosphorylation in tumors, as well as activate p53 through the DDR pathway, thereby inhibiting tumor cell repair and enhancing the radiotherapy effect of tumor cells.
Conclusion
In this study, we preliminarily determined the clinical value of the miR-125 family in HNSCC. According to the analysis results, the function of miR-125a-5p in LSCC was further verified by experiments, and we proved that miR-125a-5p can enhance the sensitivity of LSCC to radiotherapy by regulating the expression of p53. This has important implications for improving the effect of radiotherapy during treatment for laryngeal cancer.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
30 January 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s11033-024-09283-1
Abbreviations
- HNSCC:
-
Head and neck squamous cell carcinoma
- TCGA:
-
The Cancer Genome Atlas
- LSCC:
-
Laryngeal squamous cell cancer
References
Liu C, Sadat SH, Ebisumoto K, Sakai A, Panuganti BA, Ren S, Goto Y, Haft S, Fukusumi T, Ando M, Saito Y, Guo T, Tamayo P, Yeerna H, Kim W, Hubbard J, Sharabi AB, Gutkind JS, Califano JA (2020) Cannabinoids promote progression of HPV-positive head and neck squamous cell carcinoma via p38 MAPK activation. Clin Cancer Res 26(11):2693–2703. https://doi.org/10.1158/1078-0432.CCR-18-3301
Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR (2020) Head and neck squamous cell carcinoma. Nat Rev Dis Primers 6(1):92. https://doi.org/10.1038/s41572-020-00224-3
McBride S, Sherman E, Tsai CJ, et Baxi S, Aghalar J, Eng J, Zhi WI, McFarland D, Michel LS, Young R, Lefkowitz R, Spielsinger D, Zhang Z, Flynn J, Dunn L, Ho A, Riaz N, Pfister D, Lee N (2021) Randomized phase II trial of nivolumab with stereotactic body radiotherapy versus nivolumab alone in metastatic head and neck squamous cell carcinoma. J Clin Oncol 39(1):30–37. https://doi.org/10.1200/JCO.20.00290
Jia L, Mao Y, Ji Q, Dersh D, Yewdell JW, Qian SB (2020) Decoding mRNA translatability and stability from the 5’ UTR. Nat Struct Mol Biol 27(9):814–821. https://doi.org/10.1038/s41594-020-0465-x
Wu S, Shen W, Yang L, Zhu M, Zhang M, Zong F, Geng L, Wang Y, Huang T, Pan Y, Cao S, Dai J, Ma H, Wu J (2019) Genetic variations in miR-125 family and the survival of non-small cell lung cancer in Chinese population. Cancer Med 8(5):2636–2645. https://doi.org/10.1002/cam4.2073
Amiri-Dashatan N, Koushki M, Naghi-Zadeh M, Razzaghi MR, Mohaghegh Shalmani H (2021) Prognostic value of microRNA-125a/b family in patients with gastric cancer: a meta-analysis. Gastroenterol Hepatol Bed Bench 14(Suppl1):S1–S9
Piatopoulou D, Avgeris M, Marmarinos A, Xagorari M, Baka M, Doganis D, Kossiva L, Scorilas A, Gourgiotis D (2017) miR-125b predicts childhood acute lymphoblastic leukaemia poor response to BFM chemotherapy treatment. Br J Cancer 117(6):801–812. https://doi.org/10.1038/bjc.2017.256
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering CV (2019) STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47(D1):D607–D613. https://doi.org/10.1093/nar/gky1131
Lyu H, Huang J, He Z, Liu B (2018) Targeting of HER3 with functional cooperative miRNAs enhances therapeutic activity in HER2-overexpressing breast cancer cells. Biol Proced Online 20:16. https://doi.org/10.1186/s12575-018-0081-x
Zheng L, Meng X, Li X, Zhang Y, Li C, Xiang C, Xing Y, Xia Y, Xi T (2018) miR-125a-3p inhibits ERα transactivation and overrides tamoxifen resistance by targeting CDK3 in estrogen receptor-positive breast cancer. FASEB J 32(2):588–600. https://doi.org/10.1096/fj.201700461RR
Alizadeh Savareh B, Asadzadeh Aghdaie H, Behmanesh A, Bashiri A, Sadeghi A, Zali M, Shams R (2020) A machine learning approach identified a diagnostic model for pancreatic cancer through using circulating microRNA signatures. Pancreatology 20(6):1195–1204. https://doi.org/10.1016/j.pan.2020.07.399
Qi CL, Sheng JF, Huang ML, Zou Y, Wang YP, Wang F, Zeng F, Hua QQ, Chen SM (2021) Integrated analysis of deregulation microRNA expression in head and neck squamous cell carcinoma. Medicine 100(6):e24618. https://doi.org/10.1097/MD.0000000000024618
Chen L, Liu S, Li K, Qi J, Liu C, Zong L, Zhang Y, Zhao J, Zhai X, Li J, Kong X, Yang S, Liu M (2018) Evaluation of microRNA expression profiling in highly metastatic laryngocarcinoma cells. Acta Otolaryngol 138(12):1105–1111. https://doi.org/10.1080/00016489.2018.1508887
Lapa R, Barros-Filho MC, Marchi FA, Domingues M, de Carvalho GB, Drigo SA, Kowalski LP, Rogatto SR (2019) Integrated miRNA and mRNA expression analysis uncovers drug targets in laryngeal squamous cell carcinoma patients. Oral Oncol 93:76–84. https://doi.org/10.1016/j.oraloncology.2019.04.018
Yao XD, Li P, Wang JS (2021) MicroRNA differential expression spectrum and microRNA-125a-5p inhibition of laryngeal cancer cell proliferation. Exp Ther Med 21(6):545. https://doi.org/10.3892/etm.2021.9977
Lu C, El-Deiry WS (2009) Targeting p53 for enhanced radio- and chemo-sensitivity. Apoptosis 14(4):597–606. https://doi.org/10.1007/s10495-009-0330-1
Nagelkerke A, Span PN (2016) Staining against phospho-H2AX (γ-H2AX) as a marker for DNA damage and genomic instability in cancer tissues and cells. Adv Exp Med Biol 899:1–10. https://doi.org/10.1007/978-3-319-26666-4_1
Luo J, Si ZZ, Li T, Li JQ, Zhang ZQ, Chen GS, Qi HZ, Yao HL (2019) MicroRNA-146a-5p enhances radiosensitivity in hepatocellular carcinoma through replication protein A3-induced activation of the DNA repair pathway. Am J Physiol Cell Physiol 316(3):C299–C311. https://doi.org/10.1152/ajpcell.00189.2018
Lei L, Zhao X, Liu S, Cao Q, Yan B, Yang J (2019) MicroRNA-3607 inhibits the tumorigenesis of colorectal cancer by targeting DDI2 and regulating the DNA damage repair pathway. Apoptosis 24(7–8):662–672. https://doi.org/10.1007/s10495-019-01549-5
Ghoshal-Gupta S, Kutiyanawalla A, Lee BR, Ojha J, Nurani A, Mondal AK, Kolhe R, Rojiani AM, Rojiani MV (2018) TIMP-1 downregulation modulates miR-125a-5p expression and triggers the apoptotic pathway. Oncotarget 9(10):8941–8956. https://doi.org/10.18632/oncotarget.23832
Qi-Wei W, Jiang-Tao L, Li-Jun T et al (2022) MiR-125 family improves the radiosensitivity of head and neck squamous cell carcinoma. PREPRINT (Version 1). Res Square. https://doi.org/10.21203/rs.3.rs-1767318/v1
Acknowledgements
The authors thank Huijun Li of Harbin Medical University for helpful advice. A preprint has previously been published [21]. However, I have recently made further revisions to the paper.
Funding
This work was supported by the Natural Science Foundation of China (Grant No. 81272965 and 81772874) and the Natural Science Foundation of Heilongjiang Province of China (Grant No. H2017033) and the Health and Family Planning Commission of Heilongjiang Province (Grant No. 2018019) and the Scientific Research Innovation Fund of the First Affiliated Hospital of Harbin Medical University (Grant No.2021M12).
Author information
Authors and Affiliations
Contributions
J-TL and Y-NS contributed to the conception, design, and modification of the study. J-NZ, X-JZ, T-JY and Li-Jun Tan extracted the data and organized the database search. J-TL and Y-NS performed the statistical analysis. J-NZ, X-JZ, T-JY drafted the manuscript. Li-Jun Tan confirm the authenticity of all raw data. All authors contributed to the manuscript revision and read and approved the submitted version. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
Ethics approval was not required.
Patient consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s11033-024-09283-1
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, QW., Sun, YN., Tan, LJ. et al. RETRACTED ARTICLE: MiR-125 family improves the radiosensitivity of head and neck squamous cell carcinoma. Mol Biol Rep 50, 5307–5317 (2023). https://doi.org/10.1007/s11033-023-08364-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08364-x