Abstract
Background
β-thalassemia major and Niemann-Pick diseases have similar clinical and laboratory findings. We aimed to investigate the effects of sphingomyelin phosphodiesterase 1 (SMPD1) gene variants on the clinical and laboratory findings in patients with β-thalassemia major.
Methods and results
This study included 45 patients who were followed up for β-thalassemia major in our clinic. Plasma chitotriosidase, leukocyte acid sphingomyelinase, liver enzymes, ferritin, hemogram, biochemical parameters, SMPD1 gene variant analysis, cardiac T2* MRI, and liver R2 MRI were assessed in all patients. The SMPD1 gene c.132_143del, p.A46_L49del (c.108GCTGGC[4] (p.38AL[4])) (rs3838786) variant was detected in 9 of 45 (20.0%) patients. Plasma chitotriosidase, ferritin, acetyl aminotransferase, and alanine aminotransferase levels were significantly higher in patients with the gene variant than in those without (p < 0.05). Leukocyte acid sphingomyelinase levels were significantly lower in patients with the gene variant than in those without (p < 0.05).
Conclusion
These results imply that the clinical and laboratory findings and some features of disease progression in patients with β-thalassemia major are similar to those of Niemann-Pick disease. They also suggest that SMPD1 gene c.132_143del, p.A46_L49del (c.108GCTGGC[4] (p.38AL[4])) (rs3838786) variant may underlie these clinical findings in patients with β-thalassemia major.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The frequency of β-thalassemia major (β-TM) carriers in Turkey is approximately 2.1% [1]. Similarly, Niemann-Pick disease (NPD) is a common glycogen storage disease in Turkey [2]. Both diseases are autosomal recessive conditions with similar clinical presentations, including anemia, splenomegaly, and skeletal involvement [3,4,5,6]. In addition to these clinical and laboratory similarities, the genes affected in both diseases are located on the 11th chromosome [7,8,9]. There could have been a misdiagnosis of NPD in some patients who were followed up because of a β-TM diagnosis [10].
Chitotriosidase (CTD; EC 3.2.1.14) is a functional chitinase that breaks down chitin, an important component of fungal and other pathogens [11, 12]. Chitotriosidase, synthesized by specifically activated macrophages and neutrophil precursors, is encoded by a gene on chromosome 1q31; it is regulated via an unknown molecular mechanism [11, 13,14,15,16]. Acid sphingomyelinase (ASM; EC 3.1.4.12) hydrolyzes the membrane lipid sphingomyelin to phosphorylcholine and bioactive lipid ceramide. Variants in the sphingomyelin phosphodiesterase 1 (SMPD1) gene lead to the type A and B forms of lysosomal storage disorder NPD [1, 17, 18].
The fact that the genetic loci of these two diseases are located on the same chromosome suggests that there may be a similarity and relationship between them [19, 20]. Thus, we hypothesized that there may be mutations in the SMPD1 gene in patients with β-TM similar to that in patients with NPD. Variants in SMPD1 gene may contribute to liver dysfunction and hepatosplenomegaly associated with multiple blood transfusions in patients with β-TM. There are some case reports in the literature on the coexistence of β-TM and NPD [9]. However, no study has shown the role of SMPD1 variants in patients with β-TM to date. Therefore, a study of SMPD1 variants in patients with β-TM and their impact on β-TM can be beneficial in understanding the pathology of β-TM. Interestingly, the SMPD1 gene c.132_143del, p.A46_L49del (c.108GCTGGC[4] (p.38AL[4])) (rs3838786) variant is considered potentially pathogenic in the literature [20]. This study was conducted to investigate the relationship between SMPD1 gene c.132_143del, p.A46_L49del (c.108GCTGGC[4] (p.38AL[4])) (rs3838786) variant and clinical and laboratory findings of the disease in patients with β-TM.
Subjects and methods
Patients
This prospective cohort study was performed in the hematology department of Prof. Dr. Suleyman Yalcin City Hospital in Istanbul, Turkey. The study included 45 patients (25 women and 20 men) diagnosed with β-TM between the ages of 18 and 53 years. A flow diagram of the clinical trial is shown in Fig. 1. The study was initiated with 53 patients with β-TM. During the study, eight patients were excluded from the study for the following reasons: three of them did not properly administer oral iron chelation therapy, three refused to continue the study, and two had other chronic diseases (diabetes mellitus and chronic renal failure). Thus, the study was continued with 45 patients. This study was approved by the local ethics committee of the Faculty of Medicine, Medeniyet University (No. 2021/0375). Written informed consent was obtained from each study participant in accordance with the principles of the World Medical Association Declaration of Helsinki after providing a data-oriented explanation regarding the aims and scope of the study.
All patients were confirmed to have β-TM based on their clinical findings and genetics. All patients typically received regular erythrocyte suspension transfusions every three weeks and continuous oral iron chelation therapy. The patients included in the study were selected among those who were regularly followed up by our department. Patients with other hematological or chronic diseases who did not agree to participate in the study, did not attend follow-up visits, or did not regularly receive chelation treatment were excluded.
Systemic examination findings, complete blood counts, biochemical analyses, ferritin levels, vitamin levels, thyroid function test results, and imaging test results collected during routine control patient examinations were retrospectively obtained from patient files and the hospital automation system at the Thalassemia Center of Prof. Dr. Süleyman Yalçın City Hospital.
Enzyme assay
Plasma CTD activity and ASM levels were evaluated in patients with β-TM. For plasma CTD activity and ASM levels, 10 mL of blood was collected into EDTA tubes before blood transfusion.
The blood samples were rapidly transported to the laboratory. The plasma CTD activity was measured using a fluorometric method previously described [11, 13, 15]. In brief, 5 mL of plasma in an EDTA tube was incubated with 100 mL of 4-metilumbelliferyl chitotriosidase (Sigma Chemical Co., St. Louis, MO) at 37 °C in citrate/phosphate buffer (0.1/0.2 M; pH 5.2) for 15 min. The reaction was stopped by adding 2 mL of glycine/NaOH buffer (0.3 M; pH 10.6). The fluorescence of 4-methylumbelliferon was measured at 445 nm, and the results were expressed in µmol/L/h.
For the standard ASM assay, homogenates were prepared by sonication of the cell material in water [21,22,23]. Assays were performed in parallel, with and without unlabeled lysosphingomyelin. The reaction mixtures consisted of 10 µL homogenate with a standardized quantity of protein (10 µg for fibroblasts and 30 µg for leukocytes), 10 µL HMU-PC substrate, and either 10 µL LSM or 10 µL substrate buffer. The reaction mixtures were then incubated for 1 h at 37 °C (fibroblasts) or 17 h at 37 °C (leukocytes). Reactions were terminated by the addition of 200 µL stop buffer. For certain experiments, lysosphingomyelin was substituted with sphingomyelin (SM; Sigma, USA), and the effect of 0.2% Triton X-100 under these reaction conditions was studied. The fluorescence of 6-hexadecanoyl-4-methylumbelliferone was measured using a fluorimeter (Fluoro Count; Packard) with a filter set of 4-methylumbelliferone with excitation at 360 nm and emission at 460 nm. The fluorimeter was calibrated with 4-methylumbelliferone in the stop buffer.
Gene variant analysis
Gene variant analyses in this study were performed at the Genetic Diseases Evaluation Center, Duzen Laboratory Group, Ankara, Turkey. Deoxyribonucleic acid (DNA) was isolated from patient samples to analyze SMPD1 variants by Sanger sequencing and capillary electrophoresis following the relevant kit manual procedure with the MagNaPure LC 2.0 automatic isolation device (Roche diagnostic, California, USA). For DNA typing, Applied Biosystems 3500/3500xL Genetic Analyzer (Carlsbad, USA) was used [24]. Selective specific sequencing primers were designed to amplify all exons of the SMPD1 gene from the isolated DNA and distinguish it from the pseudogene. Then, PCR, amplicon control, purification, sequencing PCR, loading of the samples into the device, and data analysis were performed. For the reference sequence NG_011780.1, using the NCBI/BLAST program, all coding sequences and intronic segments were created to be +/- 20.
Statistical analyses
Statistical analysis was performed using SPSS software (Version 23.0, SPSS Inc., Chicago, IL, USA). If continuous variables were normal, they were described as the mean ± standard deviation (p > 0.05, Kolmogorov–Smirnov test or Shapiro–Wilk test (n < 30)), and if the continuous variables were not normal, they were described as the median. Continuous variables were compared using Student’s t-test or Mann–Whitney U test for parametric or nonparametric values, respectively. Categorical variables between the groups were analyzed using the chi-squared test or Fisher’s exact test. The level of statistical significance was set at p < 0.05.
Results
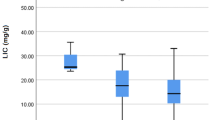
The SMPD1 gene was studied in 45 patients. Twenty-five female (55.6%) and 20 males (44.4%) patients, with a mean age of 26.8 ± 6.9 years (range: 18–53), were enrolled in the study. The laboratory findings, cardiac iron load, and hepatic iron load of the patients are presented in Table 1. The hemoglobin, platelet, LDH, total bilirubin, 25-(OH)-Vit D3, and ferritin levels were different from the reference values. Additionally, the mean plasma CTD and leukocyte ASM levels were evaluated and compared with normal reference values in some patients. The median plasma CTD activity was 91.1 (1.3–744.4) µmol/L/h and the leukocyte ASM level was 31.1 (2.3–91.8) nmol/mg/17 h. The mean plasma CTD activity was above the normal value (≥ 200 µmol/L/h) in 12 (26.7%) patients. Cardiac T2* magnetic resonance imaging (MRI) value was lower than 20 msn in 11 (24.4%) patients, and hepatic R2 value, obtained by MRI of the liver, was ≥ 7 mg/g in 13 (28.9%) patients. Furthermore, plasma ferritin levels in all patients were > 1000 ng/mL.
In this study, SMPD1 gene c.132_143del, p.A46_L49del (c.108GCTGGC[4] (p.38AL[4])) (rs3838786) variant was detected in nine (20.0%) patients. This gene has been recorded as pathological in Clin Var. In addition, some benign gene variants were detected (Table 2). No variants were detected in five patients. The patients were divided into two groups according to the SMPD1 variant results: those with or without the variant (Table 3). The mean age of the patients with and without the variant was 26.9 ± 7.6 and 25.8 ± 4.6 years, respectively. There was no statistically significant difference in patient age between the two groups (p = 0.960). Plasma CTD, leukocyte ASM, ferritin, acetyl aminotransferase (AST), and alanine aminotransferase (ALT) levels were significantly different between patients with and without the SMPD1 variant (p = 0.001, p = 0.004, p = 0.003, p = 0.009, and p = 0.017, respectively). In addition, the mean platelet volume was significantly higher in patients without the SMPD1 variant (p = 0.035) than in those with the gene variant. No statistically significant differences were observed among the other parameters. Similarly, the liver R2 results and cardiac T2* values did not differ significantly between the two groups.
Liver enzymes, plasma CTD, leukocyte ASM, and ferritin levels of patients with the SMPD1 variant are shown in Table 4. SMPD sequencing was performed on 45 patients with β-TM, and the SMPD1 gene c.132_143del, p.A46_L49del (c.108GCTGGC[4] (p.38AL[4])) (rs3838786) variant was detected in 9 of 45 (20%) patients. In the present study, four patients carrying this variant in SMPD1 were homozygous, and five were heterozygous. AST and ALT levels were above the normal reference levels in all four homozygous patients. In contrast, ALT levels were elevated in only one of the five heterozygous patients with the gene variant, whereas AST and ALT levels were within the normal range in the others. Plasma CTD levels were above 200 µmol/L/h in all nine patients with the gene variant. Leukocyte ASM levels were below the reference values in only four patients with the gene variant.
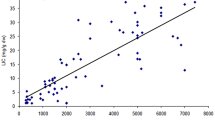
The results of the correlation studies performed in patients with the SMPD1 variant in this study are shown in Table 5. According to these results, a positive correlation was found between the plasma CTD and ferritin, AST, and ALT levels (r = 0.919, p = 0.001; r = 0.668 and p = 0.049; r = 0.968 and p = 0.001, respectively). In contrast, a negative correlation was found between leukocyte ASM levels and ferritin, AST, and ALT levels (r=-0.802, p = 0.009; r=-0.688, p = 0.048; and r=-0.779, p = 0.013, respectively).
Discussion
We analyzed SMPD1 gene variants in patients with β-TM. The SMPD1 gene c.132_143del, p.A46_L49del (c.108GCTGGC[4] (p.38AL[4])) (rs3838786) variant was detected in nine (20%) patients with β-TM. The results are novel because no such finding has been previously reported in the literature. Furthermore, we examined the effects of the SMPD1 variant on clinical and laboratory findings in patients with β-TM. We detected high levels of liver enzymes (AST and ALT), ferritin, and plasma CTD and low leukocyte ASM levels in SMPD1 variant-positive patients with β-TM.
A strong correlation exists between SMPD1 gene variants and L-ASM levels, and these gene variants are detected in patients with NPD [25, 26], as in the case of an 11-year-old patient with NPD with a rare mutation in SMPD1 [27]. However, no such studies have been conducted in patients with β-TM. In our study, L-ASM levels were lower in patients with the SMPD1 variant than in those without the gene variant. These results show that the SMPD1 variant may be important for disease progression in patients with β-TM. This study is the first to report the relationship between SMPD1 gene variants and β-TM, which should be considered in patients with β-TM.
Liver enzyme levels increase in patients with β-TM [28]. Patients with β-TM develop cirrhosis over time owing to iron accumulation [29, 30]. The role of the SMPD1 gene c.132_143del, p.A46_L49del (c.108GCTGGC[4] (p.38AL[4])) (rs3838786) variant in the development of cirrhosis remains to be studied. Recently, liver disease has been reported to be an important cause of death in patients with β-TM [31]. However, the cause of liver disease in patients with β-TM has not been fully elucidated. Life-long transfusion regimens are required to alleviate anemia in patients with β-TM. Although regular blood transfusions have greatly improved the prognosis, iron overload, especially in the liver, poses a real threat to the quality of life of patients with β-TM [32]. A genetic feature other than blood transfusion may contribute to this condition. Hepatic siderosis does not develop in all patients with β-TM despite intensive blood transfusions. In this study, liver enzyme levels were higher in patients with the SMPD1 variant than in those without. There were statistically significant differences in AST and ALT levels between patients with the SMPD1 variant and those without this gene variant. The differences in actual values between the two groups were not very large and were not considered clinically significant. The patients included in our study were selected among those who were regularly followed up by our department. Notably, the levels of liver enzymes were higher in patients with the SMPD1 variant than in those without the gene variant. This indicated that this gene variant is important for the progression of liver disease.
Variants in the SMPD1 gene are associated with liver function in patients with NPD [33,34,35]. Homozygous and compound heterozygous patients with NPD have a more severe course than heterozygous patients [36,37,38,39]. In our study, liver enzyme levels were significantly higher in four homozygous patients than in heterozygous patients. In addition, L-ASM levels were significantly lower in homozygous patients than in heterozygous patients. These results indicate that homo- or heterozygosity of the gene mutation affects the degree of disease progression.
A relationship exists between SMPD1 variants and L-ASM levels in NPD [40, 41]. However, such an association has not been reported in patients with β-TM. In the correlation tests performed in nine patients with the SMPD1 variant, plasma CTD positively correlated with ferritin, AST, and ALT, implying that as plasma CTD and ferritin levels increased in these patients, AST and ALT levels also increased. In contrast, a negative correlation was found between leukocyte ASM and ferritin, AST, and ALT. These results suggest that the SMPD1 variant causes an increase in plasma CTD, ferritin, AST, and ALT levels and a decrease in leukocyte ASM levels, similar to that in NPD.
Variant c.132_143del, p.A46_L49del (c.108GCTGGC[4] (p.38AL[4])) (rs3838786) can cause various changes in the SMPD1 gene. With this variant, valine is transformed into alanine. As a result, the protein structure is impaired; however, this is a benign change that does not impair protein synthesis. In addition, there were 12 base deletions from 32 to 143 bp in this gene. This change caused a deletion of four codons from the 46th position of the protein. This event was registered as a pathogen in ClinVar, albeit with mixed effects; 20 out of 50 cases were sick, and 30 were healthy, according to xxx. Although the effect the gene variant detected in our study has on β-TM can be open to a contradictory interpretation, we considered it to be pathological due to its clinical effect. In our opinion, the deletion mutation has a pathological effect in the clinic by disrupting protein synthesis. Similarly, in our study, we found a variant with a pathogenic effect in the clinic. This mutation should be studied further by accumulating data from more patients to determine whether it is a pathogenic gene variant.
This study had some limitations. First, a control group was not created due to financial constraints. However, we used normal reference values for leukocyte ASM and plasma CTD levels as alternatives. The second limitation was the limited number of patients.
Conclusion
In conclusion, the most important result of this study was the detection of SMPD1 gene c.132_143del, p.A46_L49del (c.108GCTGGC[4] (p.38AL[4])) (rs3838786) variant in nine (20%) patients with β-TM. Several factors play a role in liver cirrhosis in patients with β-TM. Mutations in SMPD1 in these patients may be related to increased levels of liver enzymes. Particularly, homozygous mutations may trigger this event. In addition, our findings suggest that some patients who were followed up with a diagnosis of β-TM may have a lysosomal storage disease, such as NPD. Therefore, it is necessary to refer patients with β-TM to metabolic specialists during the follow-up. We referred our patients to a metabolic specialist to monitor the possible effects of the SMPD1 variant we detected. This study is the first to examine SMPD1 variants in patients with β-TM. Importantly, this study also showed that liver enzyme and ferritin levels are high in patients with the SMPD1 variant. These results support the need for further investigation of this subject with larger series of multicenter studies.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- β-TM:
-
β-Thalassemia major
- NPD:
-
Niemann-Pick disease
- CTD:
-
Chitotriosidase
- ASM:
-
Acid sphingomyelinase
- SMPD1:
-
Sphingomyelin phosphodiesterase 1
References
Uludağ A, Uysal A, Uludağ A, Ertekin YH, Tekin M, Kütük B, Silan F, Özdemir Ö (2016) Prevalence and mutations of β-thalassemia trait and abnormal hemoglobins in premarital screening in Çanakkale province, Turkey. Balkan J Med Genet 19(1):29–34. https://doi.org/10.1515/bjmg-2016-0004
Topçu M, Aktas D, Öztoprak M, Mungan N, Yuce A, Alikasifoglu M (2017) Prospective turkish cohort study to investigate the frequency of Niemann-Pick disease type C mutations in Consanguineous families with at least one homozygous family Member. Mol Diagn Ther 21(6):643–651. https://doi.org/10.1007/s40291-017-0293-9
Seker Yilmaz B, Baruteau J, Rahim AA, Gissen P (2020) Clinical and molecular features of early Infantile Niemann pick type C disease. Int J Mol Sci 17(14):5059. https://doi.org/10.3390/ijms21145059
Langer AL, Esrick EB (2021) β-Thalassemia: evolving treatment options beyond transfusion and iron chelation. Hematol Am Soc Hematol Educ Program 2021(1):600–606. https://doi.org/10.1182/hematology
Meikle PJ, Hopwood JJ, Clague AE, Carey WF (1999) Prevalence of lysosomal storage disorders JAMA. 281:249–254. https://doi.org/10.1001/jama.281.3.249. 3
Harteveld CL, Achour A, Arkesteijn SJG, Ter Huurne J, Verschuren M, Bhagwandien-Bisoen S, Schaap R, Vijfhuizen L, El Idrissi H, Koopmann TT (2022) The hemoglobinopathies, molecular disease mechanisms and diagnostics. Int J Lab Hematol 44(1Suppl 1):28–36. https://doi.org/10.1111/ijlh.13885
Schuchman EH, Miranda SR (1997) Niemann-Pick disease: mutation update, genotype/phenotype correlations, and prospects for genetic testing. Genet Test 1(1):13–19. https://doi.org/10.1089/gte.1997.1.13
Abed Rabbo M, Khodour Y, Kaguni LS, Stiban J (2021) Sphingolipid lysosomal storage disease. Lipids Health Dis 20(1):44. https://doi.org/10.1186/s12944-021-01466-0
Yanez MJ, Marín T, Balboa E, Klein AD, Alvarez AR, Zanlungo S (2020) Finding pathogenic commonalities between Niemann-Pick type C and other lysosomal storage disorders: Opportunities for shared therapeutic interventions. Biochim Biophys Acta Mol Basis Dis 1866(10):165875. https://doi.org/10.1016/j.bbadis.2020.165875
Farahmand F, Modaresi V, Izadyar M, Mahjob F (2010) Coincidence of Niemann-Pick Disease and beta-thalassemia; a Case Report. Iran J Pediatr 20(4):483–486
Renkema GH, Boot RG, Muijsers AO, Donker-Koopman WE, Aerts JM (1995) Purification and characterization of human chitotriosidase, a novel member of the chitinase family of proteins. J Biol Chem 270(5):2198–2202. https://doi.org/10.1074/jbc.270.5.2198
Sreekantam S, Rizvi H, Brown R, Santra S, Raiman J, Vijay S, Mckiernan PJ, Gupte GL (2020) An uncommon cause of early infantile liver disease and raised chitotriosidase. JIMD Rep 54(1):22–24. https://doi.org/10.1002/jmd2.12123
De Castro-Orós I, Irún P, Cebolla JJ, Rodriguez-Sureda V, Mallén M, Pueyo MJ, Mozas P, Dominguez C, Pocoví M, Spanish NP-C, Group (2017) Assessment of plasma chitotriosidase activity, CCL18/PARC concentration and NP-C suspicion index in the diagnosis of Nieann-Pick disease type C: a prospective observational study. J Transl Med 15(1):43. https://doi.org/10.1186/s12967-017-1146-3
Degtyareva AV, Proshlyakova TY, Gautier MS, Degtyarev DN, Kamenets EA, Baydakova GV, Rebrikov DV, Zakharova EY (2019) Oxysterol/chitotriosidase based selective screening for Niemann-Pick type C in infantile cholestasis syndrome patients. BMC Med Genet 20(1):123. https://doi.org/10.1186/s12881-019-0857-0
Korolenko TA, Cherkanova MS (2009) Chitotriosidase of human macrophages and mammalian chitinases: biological functions and abnormalities in pathology. Vestn Ross Akad Med Nauk(11):39–45
Kuusk S, Sørlie M, Väljamäe P (2017) Human chitotriosidase is an endo-processive enzyme. PLoS ONE 12(1):e0171042. https://doi.org/10.1371/journal.pone.0171042
Al-Eitan L, Alqa’qa K, Amayreh W, Aljamal H, Khasawneh R, BAl-Zoubi B, Okour I, Haddad A, Haddad Y, Haddad H (2020) Novel mutations in the SMPD1 gene in jordanian children with acid sphingomyelinase deficiency (Niemann-Pick types a and B). Gene 747:144683. https://doi.org/10.1016/j.gene.2020.144683
Ota S, Noguchi A, Kondo D, Nakajima Y, Ito T, Arai H, Takahashi T, Tohoku J (2020) An early-onset neuronopathic form of acid sphingomyelinase deficiency: SMPD1 p.c133y mutation in the saposin domain of acid sphingomyelinase. Exp Med 250(1):5–11. https://doi.org/10.1620/tjem.250.5
Cao A, Galanello R (2010) Beta-thalassemia. Genet Med 12(2):61–76. https://doi.org/10.1097/GIM.0b013e3181cd68ed
Zampieri S, Filocamo M, Pianta A, Lualdi S, Gort L, Coll MJ, Sinnott R, Geberhiwot T, Bembi B, Dardis A (2016) SMPD1 mutation update: database and comprehensive analysis of published and novel variants. Hum Mutat 37(2):139–147. https://doi.org/10.1002/humu.22923
Elbin CS, Olivova P, Marashio CA, Cooper SK, Cullen E, Keutzer JM, Zhang XK (2011) The effect of preparation, storage and shipping of dried blood spots on the activity of five lysosomal enzymes. Clin Chim Acta 412(13–14):1207–1212. https://doi.org/10.1016/j.cca.2011.03.012
Gal AE, Fash FJ (1976) Synthesis of 2-n-(hexadecanoyl)-amino-4-nitrophenyl phosphorylcholine-hydroxide, a chromogenic substrate for assaying sphingomyelinase activity. Chem Phys Lipids 16(1):71–79. https://doi.org/10.1016/0009-3084(76)90015-3
Van Diggelen OP, Voznyi YV, Keulemans JLM, Schoonderwoerd K, Ledvinova J, Mengel E, Zschiesche M, Santer R, Harzer K (2005) A New fluorimetric enzyme assay for the diagnosis of Niemann–Pick A/B, with specificity of natural sphingomyelinase substrate. J Inherit Metab Dis 28(5):733–741. https://doi.org/10.1007/s10545-005-0105-y
Rhein C, Mühle C, Kornhuber J, Reichel M (2015) Alleged detrimental mutations in the SMPD1 gene in patients with niemann-pick disease. Int J Mol Sci 16(6):13649–13652. https://doi.org/10.3390/ijms160613649
Deshpande D, Gupta SK, Sarma AS, Ranganath P, Jain SJMN, Sheth J, Mistri M, Gupta N, Kabra M, Phadke SR, Girisha KM, Dua Puri R, Aggarwal S, Datar C, Mandal K, Tilak P, Muranjan M, Bijarnia-Mahay S, Rama Devi AR, Tayade NB, Ranjan A, Dalal AB (2021) Functional characterization of novel variants in SMPD1 in indian patients with acid sphingomyelinase deficiency. Hum Mutat 42(10):1336–1350. https://doi.org/10.1002/humu.24263
Ranganath P, Matta D, Bhavani GS, Wangnekar S, Jain JM, Verma IC, Kabra M, Puri RD, Danda S, Gupta N, Girisha KM, Sankar VH, Patil SJ, Ramadevi AR, Bhat M, Gowrishankar K, Mandal K, Aggarwal S, Tamhankar PM, Tilak P, Phadke SR, Dalal A (2016) Spectrum of SMPD1 mutations in asian-indian patients with acid sphingomyelinase (ASM)-deficient Niemann-Pick disease. Am J Med Genet A 170(10):2719–2730. https://doi.org/10.1002/ajmg.a.37817
Kavčič A, Homan M, Živanović M, Debeljak M, Butenko T, Drole Torkar A, Žerjav Tanšek M, Bertok S, Battelino T, Groselj U (2022) Compound heterozygote mutation in the SMPD1 gene leading to nieman-pick Disease Type A. Am J Case Rep 23:e937220. https://doi.org/10.12659/AJCR.937220
Mohammadi E, Tamaddoni A, Qujeq D, Nasseri E, Zayeri F, Zand H, Gholami M, Mir SM (2018) An investigation of the effects of curcumin on iron overload, hepcidin level, and liver function in beta-thalassemia major patients: a double-blind randomized controlled clinical trial. Phytother Res 32(9):1828–1835. https://doi.org/10.1002/ptr.6118
Al-Khabori M, Daar S, Al-Busafi SA, Al-Dhuhli H, Alumairi AA, Hassan M, Al-Rahbi S, Al-Ajmi U (2019) Noninvasive assessment and risk factors of liver fibrosis in patients with thalassemia major using shear wave elastography. Hematology 24(1):183–188. https://doi.org/10.1080/10245332.2018.1540518
Sousos N, Sinakos E, Klonizakis P, Adamidou D, Daniilidis A, Gigi E, Vetsiou E, Tsioni K, Mandala E, Vlachaki E (2018) Deferasirox improves liver fibrosis in betathalassaemia major patients. A five-year longitudinal study from a single thalassaemia centre. Br J Haematol 181(1):140–142. https://doi.org/10.1111/bjh.14509
Perifanis V, Tziomalos K, Tsatra I, Karyda S, Patsiaoura K, Athanassiou-Metaxa M (2005) Prevalence and severity of liver disease in patients with beta thalassemia major. A single-institution fifteen-year experience. Haematologica 90(8):1136–1138
Salama KM, Ibrahim OM, Kaddah AM, Boseila S, Ismail LA, Hamid MM (2015) Liver enzymes in children with beta-thalassemia Major: correlation with Iron overload and viral Hepatitis. Open Access Maced J Med Sci 3(2):287–292. https://doi.org/10.3889/oamjms.2015.059
Cassiman D, Packman S, Bembi B, Turkia HB, Al-Sayed M, Schiff M, Imrie J, Mabe P, Takahashi T, Mengel KE, Giugliani R, Cox GF (2016) Cause of death in patients with chronic visceral and chronic neurovisceral acid sphingomyelinase deficiency (Niemann-Pick disease type B and B variant): literature review and report of new cases. Mol Genet Metab 118(3):206–213. https://doi.org/10.1016/j.ymgme.2016.05.001
Ding Y, Li X, Liu Y, Hua Y, Song J, Wang L, Li M, Qin Y, Yang Y (2016) Seven novel mutations of the SMPD1 gene in four chinese patients with Niemann-Pick disease type A and prenatal diagnosis for four fetuses. Eur J Med Genet 59(4):263–268. https://doi.org/10.1016/j.ejmg.2015.11.012
Manshadi MD, Kamalidehghan B, Keshavarzi F, Aryani O, Dadgar S, Arastehkani A, Tondar M, Ahmadipour F, Meng GY, Houshmand M (2015) Four novel p.N385K, p.V36A, c.1033-1034insT and c.1417-1418delCT mutations in the sphingomyelin phosphodiesterase 1 (SMPD1) gene in patients with types a and B niemann-pick disease (NPD). Int J Mol Sci 16(4):6668–6676. https://doi.org/10.3390/ijms16046668
Baskfield A, Li R, Beers J, Zou J, Liu C, Zheng W (2019) An induced pluripotent stem cell line (TRNDi009-C) from a Niemann-Pick disease type a patient carrying a heterozygous p.L302P (c.905 T > C) mutation in the SMPD1 gene. Stem Cell Res 38:101461. https://doi.org/10.1016/j.scr.2019.101461
Cheema HA, Rasool IG, Anjum MN, Zahoor MY (2020) Mutational spectrum of SMPD1 gene in pakistani Niemann-Pick disease patients. Pak J Med Sci 36(3):479–484. https://doi.org/10.12669/pjms.36.3.467
Cerón-Rodríguez M, Vázquez-Martínez ER, García-Delgado C, Ortega-Vázquez A, Valencia-Mayoral P, Ramírez-Devars L, Arias-Villegas C, Monroy-Muñoz LE, López M, Cervantes A, Cerbón M, Morán-Barroso VF (2019) Niemann-Pick disease a or B in four pediatric patients and SMPD1 mutation carrier frequency in the mexican population. Ann Hepatol 18(4):613–619. https://doi.org/10.1016/j.aohep.2018.12.004
Naifar M, Kallel F, Hadj Kacem F, Boudabous H, Kallel R, Boudawara T, Messaoud O, Tbib N, Charfi N, Abid M, Froissart R, Messedi SH, Ayedi F (2020) Homozygous pArg610del mutation unusually Associated with severe Delay of Growth in 2 Acid Sphingomyelinase Deficiency-affected sibs. J Pediatr Hematol Oncol 42(6):e499–e502. https://doi.org/10.1097/MPH.0000000000001447
Hu J, Maegawa GHB, Zhan X, Gao X, Wang Y, Xu F, Qiu W, Han L, Gu X, Zhang H (2021) Clinical, biochemical, and genotype-phenotype correlations of 118 patients with Niemann-Pick disease types A/B. Hum Mutat 42(5):614–625. https://doi.org/10.1002/humu.24192
Ricci V, Stroppiano M, Corsolini F, Di Rocco M, Parenti G, Regis S, Grossi S, Biancheri R, Mazzotti R, Filocamo M (2004) Screening of 25 italian patients with Niemann-Pick a reveals fourteen new mutations, one common and thirteen private. Hum Mutat in SMPD1(1):105. https://doi.org/10.1002/humu.9258
Acknowledgements
We thank Ms. Cagla Sarıturk for conducting the statistical analyses. We would also like to thank the Ankara Düzen Laboratory Group for performing the gene analyses in this study.
Funding
This work was supported by the Sanofi-Genzyme Company, Turkey, under Grant (12.02.2021/01).
Author information
Authors and Affiliations
Contributions
Fadime Ersoy Dursun collected and analyzed the data, interpreted the results, and was the lead writer of the manuscript. Filiz Özen contributed to the study and interpretation of the genetic variants.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the local ethics committee of the Faculty of Medicine, Medeniyet University, Istanbul, Turkey (No: 2021/0375).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dursun, F.E., Özen, F. SMPD1 gene variants in patients with β-Thalassemia major. Mol Biol Rep 50, 3355–3363 (2023). https://doi.org/10.1007/s11033-023-08275-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08275-x