Abstract
Background
Despite their high repair capability, bone defects still present a major challenge in orthopedic tissue engineering. Osteoblast differentiation is central to the treatment of bone defects.
Methods and results
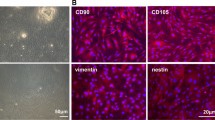
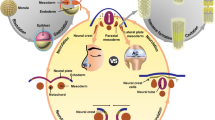
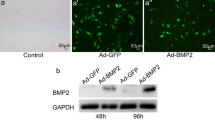
We used nasal mucosal-derived ectoderm mesenchymal stem cells (EMSCs) to promote osteogenic differentiation by co-culturing MC3T3-E1 cells. Our results showed that MC3T3-E1/EMSCs co-culture upregulated bone-related proteins and transglutaminase 2 (TG2) and increased alkaline phosphatase (ALP) activity and bone nodule formation relative to controls. Furthermore, our results showed that EMSC-derived sonic hedgehog (Shh) accounted for the enhanced MC3T3-E1 differentiation because inhibiting Shh signaling substantially reduced osteogenic differentiation.
Conclusion
Altogether, these results suggest that EMSCs differentiated into osteoblast cells and supported MC3T3-E1 differentiation. Thus, EMSCs may be a promising cell source for treating bone-related diseases.





Similar content being viewed by others
Data availability
For availability of data and materials, please contact the Xijiang Zhao for all data requests.
References
Wang W, Yeung KWK (2017) Bone grafts and biomaterials substitutes for bone defect repair: a review. Bioact Mater 2:224–247. https://doi.org/10.1016/j.bioactmat.2017.05.007
Vidal L, Kampleitner C, Brennan M, Hoornaert A, Layrolle P (2020) Reconstruction of large skeletal defects: current clinical therapeutic strategies and future directions using 3D printing. Front Bioeng Biotechnol 8:61. https://doi.org/10.3389/fbioe.2020.00061
Grecchi F, Perale G, Candotto V, Busato A, Pascali M, Carinci F (2015) Reconstruction of the zygomatic bone with smartbone®: case report. J Biol Regul Homeost Agents 29:42–47
Sohn HS, Oh JK (2019) Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater Res 23:9. https://doi.org/10.1186/s40824-019-0157-y
Gultekin BA, Bedeloglu E, Kose TE, Mijiritsky E (2016) Comparison of bone resorption rates after intraoral block bone and guided bone regeneration augmentation for the reconstruction of horizontally deficient maxillary alveolar ridges. BioMed Res Int 2016:4987437. https://doi.org/10.1155/2016/4987437
Zheng C, Chen J, Liu S, Jin Y (2019) Stem cell-based bone and dental regeneration: a view of microenvironmental modulation. Int J Oral Sci 11:23. https://doi.org/10.1038/s41368-019-0060-3
Hutchings G, Moncrieff L, Dompe C et al (2020) Bone regeneration, reconstruction and use of osteogenic cells; from basic knowledge, animal models to clinical trials. J Clin Med 9:139. https://doi.org/10.3390/jcm9010139
De Witte TM, Fratila-Apachitei LE, Zadpoor AA, Peppas NA (2018) Bone tissue engineering via growth factor delivery: from scaffolds to complex matrices. Regen Biomater 5:197–211. https://doi.org/10.1093/rb/rby013
Zhou LN, Wang JC, Zilundu PLM, Wang YQ, Guo WP, Zhang SX, Luo H, Zhou JH, Deng RD, Chen DF (2020) A comparison of the use of adipose-derived and bone marrow-derived stem cells for peripheral nerve regeneration in vitro and in vivo. Stem Cell Res Ther 11:153. https://doi.org/10.1186/s13287-020-01661-3
Shi W, Bian L, Lv D, Bi S, Dai Y, Yang K, Lu H, Zhou H, Que Y, Wang D, Zhang Z, Lu N (2020) Enhanced neural differentiation of neural stem cells by sustained release of Shh from TG2 gene-modified EMSC co-culture in vitro. Amino Acids 53:11–22. https://doi.org/10.1007/s00726-020-02918-0
Shi W, Que Y, Lv D, Bi S, Xu Z, Wang D, Zhang Z (2019) Overexpression of TG2 enhances the differentiation of ectomesenchymal stem cells into neuronlike cells and promotes functional recovery in adult rats following spinal cord injury. Mol Med Rep 20:2763–2773. https://doi.org/10.3892/mmr.2019.10502
Shi W, Wang Z, Bian L, Wu Y, HuiYa M, Zhou Y, Zhang Z, Wang Q, Zhao P, Lu X (2022) Periodic heat stress licenses EMSC differentiation into osteoblasts via YAP signaling pathway activation. Stem Cells Int. https://doi.org/10.1155/2022/3715471
Shi W, Zhang X, Bian L, Dai Y, Wang Z, Zhou Y, Yu S, Zhang Z, Zhao P, Tang H, Wang Q, Lu X (2022) Alendronate crosslinked chitosan/polycaprolactone scaffold for bone defects repairing. Int J Biol Macromol 204:441–456. https://doi.org/10.1016/j.ijbiomac.2022.02.007
Martino MM, Briquez PS, Maruyama K, Hubbell JA (2015) Extracellular matrix-inspired growth factor delivery systems for bone regeneration. Adv Drug Deliv Rev 94:41–52. https://doi.org/10.1016/j.addr.2015.04.007
Wang L, Liu S, Zhao Y, Liu D, Liu Y, Chen C, Karray S, Shi S, Jin Y (2015) Osteoblast-induced osteoclast apoptosis by fas ligand/FAS pathway is required for maintenance of bone mass. Cell Death Differ 22:1654–1664. https://doi.org/10.1038/cdd.2015.14
Long J, Tokhunts R, Old WM, Houel S, Rodgriguez-Blanco J, Singh S, Schilling N, Capobianco AJ, Ahn NG, Robbins DJ (2015) Identification of a family of fatty-acid-speciated sonic hedgehog proteins, whose members display differential biological properties. Cell Rep 10:1280–1287. https://doi.org/10.1016/j.celrep.2015.01.058
Chakrabarti J, Dua-Awereh M, Schumacher M, Engevik A, Hawkins J, Helmrath MA, Zavros Y (2022) Sonic Hedgehog acts as a macrophage chemoattractant during regeneration of the gastric epithelium. NPJ Regen Med 7:3. https://doi.org/10.1038/s41536-021-00196-2
Zavala G, Prieto CP, Villanueva AA, Palma V (2017) Sonic hedgehog (SHH) signaling improves the angiogenic potential of Wharton’s jelly-derived mesenchymal stem cells (WJ-MSC). Stem Cell Res Ther 8:203. https://doi.org/10.1186/s13287-017-0653-8
Pang P, Shimo T, Takada H, Matsumoto K, Yoshioka N, Ibaragi S, Sasaki A (2015) Expression pattern of sonic hedgehog signaling and calcitonin gene-related peptide in the socket healing process after tooth extraction. Biochem Biophys Res Commun 467:21–26. https://doi.org/10.1016/j.bbrc.2015.09.139
Deng W, Shao F, He Q et al (2019) EMSCs build an all-in-one niche via cell–cell lipid raft assembly for promoted neuronal but suppressed astroglial differentiation of neural stem cells. Adv Mater 31:1806861. https://doi.org/10.1002/adma.201806861
Kreke MR, Sharp LA, Woo Lee Y, Goldstein AS (2008) Effect of intermittent shear stress on mechanotransductive signaling and osteoblastic differentiation of bone marrow stromal cells. Tissue Eng A 14:529–537. https://doi.org/10.1089/tea.2007.0068
Zhou Y, Chen F, Ho S, Woodruff M, Lim T, Hutmacher D (2007) Combined marrow stromal cell-sheet techniques and high-strength biodegradable composite scaffolds for engineered functional bone grafts. Biomaterials 28:814–824. https://doi.org/10.1016/j.biomaterials.2006.09.032
Kim YS, Majid M, Melchiorri AJ, Mikos AG (2018) Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioeng Transl Med 4:83–95. https://doi.org/10.1002/btm2.10110
Lai TS, Lin CJ, Greenberg CS (2016) Role of tissue transglutaminase-2 (TG2)-mediated aminylation in biological processes. Amino Acids 49:501–515. https://doi.org/10.1007/s00726-016-2270-8
Fukunishi T, Ong CS, He YJ, Inoue T, Zhang H, Steppan J, Matsushita H, Johnson J, Santhanam L, Hibino N (2021) Fast-degrading tissue-engineered vascular grafts lead to increased extracellular matrix cross-linking enzyme expression. Tissue Eng A 27:1368–1375. https://doi.org/10.1089/ten.tea.2020.0266
Shi W, Que Y, Zhang X et al (2021) Functional tissue-engineered bone-like graft made of a fibrin scaffold and TG2 gene-modified EMSCs for bone defect repair. NPG Asia Mater 13:28. https://doi.org/10.1038/s41427-021-00297-w
Jia Q, Jiang W, Ni L (2015) Down-regulated non-coding RNA (lncRNA-ANCR) promotes osteogenic differentiation of periodontal ligament stem cells. Arch Oral Biol 60:234–241. https://doi.org/10.1016/j.archoralbio.2014.10.007
Yuan X, Cao J, He X, Serra R, Qu J, Cao X, Yang S (2016) Ciliary IFT80 balances canonical versus non-canonical hedgehog signalling for osteoblast differentiation. Nat Commun 7:11024. https://doi.org/10.1038/ncomms11024
Lv T, Shen L, Yang L, Diao W, Yang Z, Zhang Y, Yu S, Li Y (2018) Polydatin ameliorates dextran sulfate sodium-induced colitis by decreasing oxidative stress and apoptosis partially via sonic hedgehog signaling pathway. Int Immunopharmacol 64:256–263. https://doi.org/10.1016/j.intimp.2018.09.009
Hosoya A, Shalehin N, Takebe H, Fujii S, Seki Y, Mizoguchi T, Shimo T, Iijima M, Irie K (2020) Stem cell properties of Gli1-positive cells in the periodontal ligament. J Oral Biosci 62:299–305. https://doi.org/10.1016/j.job.2020.08.002
Doro DH, Grigoriadis AE, Liu KJ (2017) Calvarial suture-derived stem cells and their contribution to cranial bone repair. Front Physiol 8:956. https://doi.org/10.3389/fphys.2017.00956
Peng Y, Huang D, Liu S, Li J, Qing X, Shao Z (2020) Biomaterials-induced stem cells specific differentiation into intervertebral disc lineage cells. Front Bioeng Biotechnol 8:56. https://doi.org/10.3389/fbioe.2020.00056
Li LT, Jiang G, Chen Q, Zheng JN (2015) Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol Med Rep 11:1566–1572. https://doi.org/10.3892/mmr.2014.2914
Qin S, Sun D, Li H, Li X, Pan W, Yan C, Tang R, Liu X (2015) The effect of SHH-Gli signaling pathway on the synovial fibroblast proliferation in rheumatoid arthritis. Inflammation 39:503–512. https://doi.org/10.1007/s10753-015-0273-3
Acknowledgements
We thank the University Ethics Committee for their kind guidance in the animal experiments.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81571830). This study was also supported by the Special fund project for the development of health science and technology in Nanjing (Grant No. YKK19129) and (Grants No. JYL20180040).
Author information
Authors and Affiliations
Contributions
Study concept: LB and YW; data curation: JW; formal analysis: LB and YW; funding acquisition: PZ and XZ; methodology: JW; resources: YW, JW, and LB; writing and original draft: PZ and XZ; writing, review and editing: LB and XZ. All authors approved the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Ethical approval
All of the animal procedures were approved by the Jiangnan University Animal Care and Ethics Committee, and the International Guidelines for Animal Research were strictly followed in this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bian, L., Wu, Y., Wu, J. et al. Ectoderm mesenchymal stem cells promote osteogenic differentiation of MC3T3-E1 cells by targeting sonic hedgehog signaling pathway. Mol Biol Rep 50, 1293–1302 (2023). https://doi.org/10.1007/s11033-022-08022-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-08022-8