Abstract
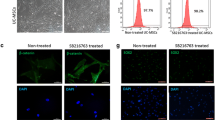
As a promising cell therapy, neural crest-derived ectoderm mesenchymal stem cells (EMSCs) secrete high amounts of extracellular matrix (ECM) and neurotrophic factors, promoting neural stem cell (NSC) differentiation into neuronal lineages and aiding tissue regeneration. Additionally, the forced overexpression of secreted proteins can increase the therapeutic efficacy of the secretome. Tissue transglutaminase (TG2) is a ubiquitously expressed member of the transglutaminase family of calcium-dependent crosslinking enzymes, which can stabilize the ECM, inducing smart or living biomaterial to stimulate differentiation and enhance the neurogenesis of NSCs. In this study, we examined the neuronal differentiation of NSCs induced by TG2 gene-modified EMSCs (TG2-EMSCs) in a co-culture model directly. Two weeks after initiating differentiation, levels of the neuronal markers, tubulin beta 3 class III and growth-associated protein 43, were higher in NSCs in the TG2-EMSC co-culture group and those of the astrocytic marker glial fibrillary acidic protein were lower, compared with the control group. These results were confirmed by immunofluorescence, and laminin, fibronectin and sonic hedgehog (Shh) contributed to this effect. The results of western blot analysis and the enzyme-linked immunoassay showed that after TG2-EMSCs were co-cultured for 2 weeks, they expressed much higher levels of Shh than the control group. Moreover, the sustained release of Shh was observed in the TG2-EMSC co-culture group. Overall, our findings indicate that EMSCs can induce the differentiation of NSCs, of which TG2-EMSCs can promote the differentiation of NSCs compared with EMSCs.










Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W (2017) Cell transplantation therapy for spinal cord injury. Nat Neurosci 20:637–647
Belkin AM (2011) Extracellular TG2: emerging functions and regulation. FEBS J 278:4704–4716
Bhate D, Penick CA, Ferry LA, Lee C (2019) Classification and selection of cellular materials in mechanical design: engineering and biomimetic approaches. Designs 3:19
Cardozo A, Ielpi M, Gomez D (2010) Differential expression of Shh and BMP signaling in the potential conversion of human adipose tissue stem cells into neuron-like cells in vitro[J]. Gene Expr J Liver Res 14(6):307–319
Chang C, Lahti T, Tanaka T et al (2018) Egg proteins: fractionation, bioactive peptides and allergenicity[J]. J Sci Food Agric 98(15):5547–5558
Cui X, Zhang X, Bu H, Liu N, Li H, Guan X, Yan H, Wang Y, Zhang H, Ding Y (2017) Shear stress-mediated changes in the expression of complement regulatory protein CD59 on human endothelial progenitor cells by ECM-integrinαVβ3-F-actin pathway in vitro. Biochem Biophys Res Commun 494:416–421
Deng W, Shao F, He Q, Wang Q, Shi W, Yu Q, Cao X, Feng C, Bi S, Chen J (2019) EMSCs build an all-in-one niche via cell-cell lipid raft assembly for promoted neuronal but suppressed astroglial differentiation of neural stem cells. Adv Mater 31:1806861
Gao S, Guo X, Zhao S, Jin Y, Zhou F, Yuan P, Cao L, Wang J, Qiu Y, Sun C (2019) Differentiation of human adipose-derived stem cells into neuron/motoneuron-like cells for cell replacement therapy of spinal cord injury. Cell Death Dis 10:1–15
Gomes ED, Rocha LA, Assunção-Silva RC et al (2020) Cell therapies for spinal cord injury regeneration. In: Perale G, Rossi F (eds) Spinal cord injury (SCI) repair strategies. Woodhead Publishing, pp 157–186
Hasan H, Wu P (2020) Insights into exogenous, endogenous and combination therapies of neural stem cells in spinal cord injury. Front Stem Cell Regen Med Res 9(9):1–75
Jia Y, Wu D, Zhang R et al (2014) Bone marrow-derived mesenchymal stem cells expressing the Shh transgene promotes functional recovery after spinal cord injury in rats[J]. Neurosci Lett 573:46–51
Klobučar M, Grbčić P, Pavelić SK, Jonjić N, Visentin S, Sedić M (2018) Acid ceramidase inhibition sensitizes human colon cancer cells to oxaliplatin through downregulation of transglutaminase 2 and β1 integrin/FAK—mediated signalling. Biochem Biophys Res Commun 503:843–848
Lai T-S, Lin C-J, Greenberg CS (2017) Role of tissue transglutaminase-2 (TG2)-mediated aminylation in biological processes. Amino Acids 49:501–515
Lestanova Z, Bacova Z, Bakos J (2016) Mechanisms involved in the regulation of neuropeptide-mediated neurite outgrowth: a minireview. Endocr Regul 50:72–82
Liu S, Chen Z (2019) Employing Endogenous NSCs to Promote Recovery of Spinal Cord Injury. Stem Cells Int 2019:1958631. https://doi.org/10.1155/2019/1958631
Liu H, Xu X, Tu Y, Chen K, Song L, Zhai J, Chen S, Rong L, Zhou L, Wu W (2020) Engineering microenvironment for endogenous neural regeneration after spinal cord injury by reassembling extracellular matrix. ACS Appl Mater Interfaces 12:17207–17219
Lu P, Ahmad R, Tuszynski MH (2016) Neural stem cells for spinal cord injury. In: Tuszynski M (ed) Translational Neuroscience. Springer, Boston, MA. https://doi.org/10.1007/978-1-4899-7654-3_16
Murr LE (2019) Strategies for creating living, additively manufactured, open-cellular metal and alloy implants by promoting osseointegration, osteoinduction and vascularization: an overview. J Mater Sci Technol 35:231–241
Nakamura M, Okano H, Ogawa Y et al (2003) Transplantation of neural stem cells promote axonal growth and functional recovery after spinal cord injury in neonate rats. Spine Journal Meeting Abstracts 31–41
Nguyen PQ, Courchesne NMD, Duraj-Thatte A et al (2018) Engineered living materials: prospects and challenges for using biological systems to direct the assembly of smart materials[J]. Adv Mater 30(19):1704847
Papa S, Pizzetti F, Perale G, Veglianese P, Rossi F (2020) Regenerative medicine for spinal cord injury: focus on stem cells and biomaterials. Expert Opin Biol Ther 20(10):1203–1213
Park D, Choi SS, Ha K-S (2010) Transglutaminase 2: a multi-functional protein in multiple subcellular compartments. Amino Acids 39:619–631
Rabinstein AA (2020) Traumatic spinal cord injury. In: Rabinstein AA (ed) Neurological emergencies. Springer, Cham, pp 271–280
Scandella V, Knobloch M (2019) Sensing the environment: extracellular lactate levels control adult neurogenesis. Cell Stem Cell 25:729–731
Shao A, Tu S, Lu J, Zhang J (2019) Crosstalk between stem cell and spinal cord injury: pathophysiology and treatment strategies. Stem Cell Res Ther 10:238
Shi W, Que Y, Lv D, Bi S, Xu Z, Wang D, Zhang Z (2019a) Overexpression of TG2 enhances the differentiation of ectomesenchymal stem cells into neuron-like cells and promotes functional recovery in adult rats following spinal cord injury. Mol Med Rep 20:2763–2773
Shi W, Bi S, Dai Y, Yang K, Zhao Y, Zhang Z (2019b) Clobetasol propionate enhances neural stem cell and oligodendrocyte differentiation. Exp Ther Med 18:1258–1266
Xu C-J, Wang J-L, Jin W-L (2015) The neural stem cell microenvironment: focusing on axon guidance molecules and myelin-associated factors. J Mol Neurosci 56:887–897
Yang B, Zhang F, Cheng F, Ying L, Wang C, Shi K, Wang J, Xia K, Gong Z, Huang X (2020a) Strategies and prospects of effective neural circuits reconstruction after spinal cord injury. Cell Death Dis 11:1–14
Yang W, Wang Z, Zhang J, Yang K, Lu C, Cui X, Lu H, Cao S, Chen Q, Lu X (2020b) Fibrin scaffolds embedded with sonic hedgehog/chitosan microspheres for recovery of spinal cord injury in rats. Mater Express 10:437–445
Zhang Z, He Q, Deng W et al (2015) Nasal ectomesenchymal stem cells: multi-lineage differentiation and transformation effects on fibrin gels[J]. Biomaterials 49:57–67
Zhang Z, Li Z, Deng W, He Q, Wang Q, Shi W, Chen Q, Yang W, Spector M, Gong A (2016) Ectoderm mesenchymal stem cells promote differentiation and maturation of oligodendrocyte precursor cells. Biochem Biophys Res Commun 480:727–733
Zhang Q, Shi B, Ding J, Yan L, Thawani JP, Fu C, Chen X (2019) Polymer scaffolds facilitate spinal cord injury repair. Acta Biomater 88:57–77
Zhao Y, Xiao Z, Chen B, Dai J (2017) The neuronal differentiation microenvironment is essential for spinal cord injury repair. Organogenesis 13:63–70
Zhu Y, Uezono N, Yasui T, Nakashima K (2018) Neural stem cell therapy aiming at better functional recovery after spinal cord injury. Dev Dyn 247:75–84
Funding
This study was supported by the National Natural Science Foundation of China (Grants No. 81571830). This study was also supported by the Special fund project for the development of health science and technology in Nanjing (Grants No. YKK19129) and Jiangsu University Clinical/Medical Science and Technology Development Fund Projectproject number (Grants No. JYL20180040).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
All of the animal procedures were approved by the Jiangsu University Animal Care and Ethics Committee, and the International Guidelines for Animal Research were strictly followed in this study.
Informed consent
All authors listed have contributed to conception, design, gathering, analysis or interpretation of data and have contributed to the writing and intellectual content of the article. All authors gave informed consent to the submission of this manuscript.
Additional information
Handling Editor: T. Harkany.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shi, W., Bian, L., Lv, D. et al. Enhanced neural differentiation of neural stem cells by sustained release of Shh from TG2 gene-modified EMSC co-culture in vitro. Amino Acids 53, 11–22 (2021). https://doi.org/10.1007/s00726-020-02918-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-020-02918-0