Abstract
Background
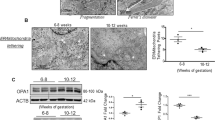
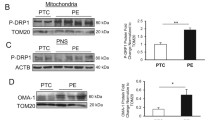
Gestational diabetes mellitus (GDM) is a metabolic complication that affects millions of pregnant women in the world. Placental tissue function is endangered by hyperglycemia during GDM, which is correlated to increased incidences of pregnancy complications. Recently we showed that due to a significant decrease in mitochondrial fusion, mitochondrial dynamics equilibrium is altered in placental tissues from GDM patients. Evidence for the role of reduced mitochondrial fusion in the disruption of mitochondrial function in placental cells is limited.
Methods and Results
Here we show that chemical inhibition of mitochondrial fission in cultured placental trophoblast cells leads to an increase in mitochondrial fusion and improves the physiological state of these cells and hence, their capacity to cope in a hyperglycemic environment. Specifically, mitochondrial fission inhibition led to a reduction in reactive oxygen species (ROS) generation, mitochondrial unfolded protein marker expressions, and mitochondrial depolarization. It supported the increase in mitochondrial antioxidant enzyme expressions as well. Mitochondrial fission inhibition also increases the placental cell insulin sensitivity during hyperglycemia.
Conclusion
Our results suggest that mitochondrial fusion/fission equilibrium is critical for placental cell function and signify the therapeutic potential of small molecule inhibitors of fission during GDM.






Similar content being viewed by others
References
Association AD (2014) Diagnosis and classification of diabetes mellitus. Diabetes Care 37(Supplement 1):S81–S90
Neiger R (2017) Long-term effects of pregnancy complications on maternal health: a review. J Clin Med 6(8):76
Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC (2000) Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 49(12):2208–2211
Langer O, Yogev Y, Most O, Xenakis EM (2005) Gestational diabetes: the consequences of not treating. Am J Obstet Gynecol 192(4):989–997
Catalano PM, Kirwan JP, Haugel-de Mouzon S, King J (2003) Gestational diabetes and insulin resistance: role in short-and long-term implications for mother and fetus. J Nutr 133(5):1674S–1683S
López-Tello J, Pérez-García V, Khaira J, Kusinski LC, Cooper WN, Andreani A, Grant I, de Liger EF, Lam BY, Hemberger M (2019) Fetal and trophoblast PI3K p110α have distinct roles in regulating resource supply to the growing fetus in mice. Elife 8:e45282
Sandovici I, Hoelle K, Angiolini E, Constância M (2012) Placental adaptations to the maternal–fetal environment: implications for fetal growth and developmental programming. Reprod Biomed Online 25(1):68–89
Desforges M, Sibley CP (2009) Placental nutrient supply and fetal growth. Int J Dev Biol 54(2–3):377–390
Burton GJ, Yung HW, Murray AJ (2017) Mitochondrial–endoplasmic reticulum interactions in the trophoblast: stress and senescence. Placenta 52:146–155
Martinez F, Olvera-Sanchez S, Esparza-Perusquia M, Gomez-Chang E, Flores-Herrera O (2015) Multiple functions of syncytiotrophoblast mitochondria. Steroids 103:11–22
Westermann B (2010) Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11(12):872–884
Youle RJ, Van Der Bliek AM (2012) Mitochondrial fission, fusion, and stress. Science 337(6098):1062–1065
Galloway CA, Yoon Y (2013) Mitochondrial morphology in metabolic diseases. Antioxid Redox Sign 19(4):415–430
Otera H, Mihara K (2011) Molecular mechanisms and physiologic functions of mitochondrial dynamics. J Biochem 149(3):241–251
Yoon Y, Galloway CA, Jhun BS, Yu T (2011) Mitochondrial dynamics in diabetes. Antioxid Redox Sign 14(3):439–457
Gerber PA, Rutter GA (2017) The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid Redox Sign 26(10):501–518
Wu G, Xiong Q, Wei X, Wang Y, Hu X, He G, Liu L, Lai Q, Dai Z, Anushesh D (2019) Mitochondrial unfolded protein response gene CLPP changes mitochondrial dynamics and affects mitochondrial function. PeerJ 7:e7209
Wang L, Ishihara T, Ibayashi Y, Tatsushima K, Setoyama D, Hanada Y, Takeichi Y, Sakamoto S, Yokota S, Mihara K (2015) Disruption of mitochondrial fission in the liver protects mice from diet-induced obesity and metabolic deterioration. Diabetologia 58(10):2371–2380
Lin H-Y, Weng S-W, Chang Y-H, Su Y-J, Chang C-M, Tsai C-J, Shen F-C, Chuang J-H, Lin T-K, Liou C-W (2018) The causal role of mitochondrial dynamics in regulating insulin resistance in diabetes: link through mitochondrial reactive oxygen species. Oxidative Medicine and Cellular Longevity 2018
Hulme CH, Nicolaou A, Murphy SA, Heazell AE, Myers JE, Westwood M (2019) The effect of high glucose on lipid metabolism in the human placenta. Sci Rep 9(1):1–9
Hulme C, Stevens A, Dunn W, Heazell AE, Hollywood K, Begley P, Westwood M, Myers J (2018) Identification of the functional pathways altered by placental cell exposure to high glucose: lessons from the transcript and metabolite interactome. Sci Rep 8(1):1–11
He M-y, Wang G, Han S-s, Jin Y, Li H, Wu X, Ma Z-l, Cheng X, Tang X, Yang X (2016) Nrf2 signalling and autophagy are involved in diabetes mellitus-induced defects in the development of mouse placenta. Open biology 6(7):160064
Wada J, Nakatsuka A (2016) Mitochondrial dynamics and mitochondrial dysfunction in diabetes. Acta Med Okayama 70(3):151–158
Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR (2008) Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell 14(2):193–204
Wang Q, Zhang M, Torres G, Wu S, Ouyang C, Xie Z, Zou M-H (2017) Metformin suppresses diabetes-accelerated atherosclerosis via the inhibition of Drp1-mediated mitochondrial fission. Diabetes 66(1):193–205
Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T (2006) Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell 10(6):839–850
Gao D, Zhang L, Dhillon R, Hong T-T, Shaw RM, Zhu J (2013) Dynasore protects mitochondria and improves cardiac lusitropy in Langendorff perfused mouse heart.PLoS One8 (4)
Qi X, Qvit N, Su Y-C, Mochly-Rosen D (2013) A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J Cell Sci 126(3):789–802
Su Y-C, Qi X (2013) Inhibition of excessive mitochondrial fission reduced aberrant autophagy and neuronal damage caused by LRRK2 G2019S mutation. Hum Mol Genet 22(22):4545–4561
Zhan L, Cao H, Wang G, Lyu Y, Sun X, An J, Wu Z, Huang Q, Liu B, Xing J (2016) Drp1-mediated mitochondrial fission promotes cell proliferation through crosstalk of p53 and NF-κB pathways in hepatocellular carcinoma. Oncotarget 7(40):65001
Kolac UK, Eken MK, Ünübol M, Yalcin GD, Yalcin A (2021) The effect of gestational diabetes on the expression of mitochondrial fusion proteins in placental tissue. Placenta 115:106–114
Easton ZJ, Luo X, Li L, Regnault TR (2022) The impact of hyperglycemia upon BeWo trophoblast cell metabolic function: A multi-OMICS and functional metabolic analysis. bioRxiv
Inadera H, Tachibana S, Takasaki I, Tatematsu M, Shimomura A (2010) Hyperglycemia perturbs biochemical networks in human trophoblast BeWo cells. Endocr J 57(7):567–577
Yalcin A, Şarkici G, Kolaç UK (2020) PKR inhibitors suppress endoplasmic reticulum stress and subdue glucolipotoxicitymediated impairment of insulin secretion in pancreatic beta cells. Turkish J Biology 44(2):93–102
Bordt EA, Clerc P, Roelofs BA, Saladino AJ, Tretter L, Adam-Vizi V, Cherok E, Khalil A, Yadava N, Shealinna XG (2017) The putative Drp1 inhibitor mdivi-1 is a reversible mitochondrial complex I inhibitor that modulates reactive oxygen species. Dev Cell 40(6):583–594 e586
Wappler EA, Institoris A, Dutta S, Katakam PV, Busija DW (2013) Mitochondrial dynamics associated with oxygen-glucose deprivation in rat primary neuronal cultures.PloS one8 (5)
Chung C-L, Sheu J-R, Liu H-E, Chang S-C, Chou Y-C, Chen W-L, Chou D-S, Hsiao G (2009) Dynasore, a dynamin inhibitor, induces PAI-1 expression in MeT-5A human pleural mesothelial cells. Am J Respir Cell Mol Biol 40(6):692–700
Girard E, Paul JL, Fournier N, Beaune P, Johannes L, Lamaze C, Védie B (2011) The dynamin chemical inhibitor dynasore impairs cholesterol trafficking and sterol-sensitive genes transcription in human HeLa cells and macrophages. PloS one 6 (12)
Joshi AU, Saw NL, Vogel H, Cunnigham AD, Shamloo M, Mochly-Rosen D (2018) Inhibition of Drp1/Fis1 interaction slows progression of amyotrophic lateral sclerosis.EMBO molecular medicine10 (3)
Kumari S, Anderson L, Farmer S, Mehta SL, Li PA (2012) Hyperglycemia alters mitochondrial fission and fusion proteins in mice subjected to cerebral ischemia and reperfusion. Translational stroke research 3(2):296–304
Yu T, Sheu S-S, Robotham JL, Yoon Y (2008) Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovascular Res 79(2):341–351
Xu Z, Zhang L, Li X, Jiang Z, Sun L, Zhao G, Zhou G, Zhang H, Shang J, Wang T (2015) Mitochondrial fusion/fission process involved in the improvement of catalpol on high glucose-induced hepatic mitochondrial dysfunction. Acta Biochim Biophys Sin 47(9):730–740
Cerqueira FM, Chausse B, Baranovski BM, Liesa M, Lewis EC, Shirihai OS, Kowaltowski AJ (2016) Diluted serum from calorie-restricted animals promotes mitochondrial β‐cell adaptations and protect against glucolipotoxicity. FEBS J 283(5):822–833
Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA (2011) Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 124(4):444–453
Brown GC, Murphy MP, Jornayvaz FR, Shulman GI (2010) Regulation of mitochondrial biogenesis. Essays Biochem 47:69–84
Guo C, Wang J, Jing L, Ma R, Liu X, Gao L, Cao L, Duan J, Zhou X, Li Y (2018) Mitochondrial dysfunction, perturbations of mitochondrial dynamics and biogenesis involved in endothelial injury induced by silica nanoparticles. Environ Pollut 236:926–936
Kiritoshi S, Nishikawa T, Sonoda K, Kukidome D, Senokuchi T, Matsuo T, Matsumura T, Tokunaga H, Brownlee M, Araki E (2003) Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: potential role in diabetic nephropathy. Diabetes 52(10):2570–2577
Nishikawa T, Edelstein D, Du XL, Yamagishi S-i, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes H-P (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404(6779):787–790
Sivitz WI, Yorek MA (2010) Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Sign 12(4):537–577
Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin C-T, Price JW, Kang L, Rabinovitch PS, Szeto HH (2009) Mitochondrial H 2 O 2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119(3):573–581
Yu T, Robotham JL, Yoon Y (2006) Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proceedings of the National Academy of Sciences 103 (8):2653–2658
Haynes CM, Fiorese CJ, Lin Y-F (2013) Evaluating and responding to mitochondrial dysfunction: the mitochondrial unfolded-protein response and beyond. Trends Cell Biol 23(7):311–318
Deepa SS, Bhaskaran S, Ranjit R, Qaisar R, Nair BC, Liu Y, Walsh ME, Fok WC, Van Remmen H (2016) Down-regulation of the mitochondrial matrix peptidase ClpP in muscle cells causes mitochondrial dysfunction and decreases cell proliferation. Free Radical Bio Med 91:281–292
Becker C, Kukat A, Szczepanowska K, Hermans S, Senft K, Brandscheid CP, Maiti P, Trifunovic A (2018) CLPP deficiency protects against metabolic syndrome but hinders adaptive thermogenesis. EMBO Rep 19(5):e45126
Hall L, Martinus RD (2013) Hyperglycaemia and oxidative stress upregulate HSP60 & HSP70 expression in HeLa cells. Springerplus 2(1):1–10
Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ (2001) The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1(4):515–525
Clerc P, Ge S, Hwang H, Waddell J, Roelofs B, Karbowski M, Sesaki H, Polster B (2014) Drp 1 is dispensable for apoptotic cytochrome c release in primed MCF 10 A and fibroblast cells but affects Bcl-2 antagonist‐induced respiratory changes. Br J Pharmacol 171(8):1988–1999
Sugioka R, Shimizu S, Tsujimoto Y (2004) Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem 279(50):52726–52734
Holland O, Nitert MD, Gallo LA, Vejzovic M, Fisher JJ, Perkins AV (2017) Placental mitochondrial function and structure in gestational disorders. Placenta 54:2–9
Jheng H-F, Tsai P-J, Guo S-M, Kuo L-H, Chang C-S, Su I-J, Chang C-R, Tsai Y-S (2012) Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol 32(2):309–319
Chan DC (2012) Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet 46:265–287
Liesa M, Shirihai OS (2013) Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metabol 17(4):491–506
Hastie R, Lappas M (2014) The effect of pre-existing maternal obesity and diabetes on placental mitochondrial content and electron transport chain activity. Placenta 35(9):673–683
Fisher JJ, Vanderpeet CL, Bartho LA, McKeating DR, Cuffe JS, Holland OJ, Perkins AV (2021) Mitochondrial dysfunction in placental trophoblast cells experiencing gestational diabetes mellitus. J Physiol 599(4):1291–1305
Prasun P (2020) Mitochondrial dysfunction in metabolic syndrome. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 1866:16583810
Das M, Sauceda C, Webster NJ (2021) Mitochondrial dysfunction in obesity and reproduction. Endocrinology 162(1):bqaa158
Wang J, Lin X, Zhao N, Dong G, Wu W, Huang K, Fu J (2022) Effects of Mitochondrial Dynamics in the Pathophysiology of Obesity. Front Bioscience-Landmark 27(3):107
Mandò C, Anelli GM, Novielli C, Panina-Bordignon P, Massari M, Mazzocco MI, Cetin I (2018) Impact of obesity and hyperglycemia on placental mitochondria. Oxidative medicine and cellular longevity 2018
Wu B, Chen Y, Clarke R, Akala E, Yang P, He B, Gao H (2022) AMPK Signaling Regulates Mitophagy and Mitochondrial ATP Production in Human Trophoblast Cell Line BeWo. Front Bioscience-Landmark 27(4):118
Wasilewski M, Semenzato M, Rafelski SM, Robbins J, Bakardjiev AI, Scorrano L (2012) Optic atrophy 1-dependent mitochondrial remodeling controls steroidogenesis in trophoblasts. Curr Biol 22(13):1228–1234
Funding
This research was supported by the Scientific and Technological Research Council of Turkey (TUBİTAK Project No: 120S283).
Author information
Authors and Affiliations
Contributions
U.K.K. design and conduction of the research, analysis and interpretation of the data and writing of the initial draft of the manuscript. A.Y. and G.D.Y. analysis and interpretation of the data and writing of the initial draft of the manuscript. All the authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.


Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kolac, U.K., Donmez Yalcin, G. & Yalcin, A. Chemical inhibition of mitochondrial fission improves insulin signaling and subdues hyperglycemia induced stress in placental trophoblast cells. Mol Biol Rep 50, 493–506 (2023). https://doi.org/10.1007/s11033-022-07959-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07959-0