Abstract
Introduction
Breeding studies are commonly conducted to develop new cultivars with high yield levels and improved quality traits. Chemically-induced mutations are used to create genetic variations in wheat genomes. Various physical and chemical mutagens are used to increase frequency of mutations and facilitate the selection processes. Sodium azide (SA) is largely employed to induce mutations of the genes regulating essential traits. Such mutations may also elucidate gene functions of the mutant phenotypes. Present experiments were conducted to investigate potential use of conventional chemical mutagenesis technique through SA for mature embryo culture in wheat.
Methods and results
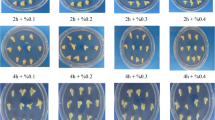
Sodium azide mutagenesis was experimented with 4 treatment durations (1, 2, 3 and 4 h) and 5 treatment concentrations (0, 1, 2, 3 and 4 mM). Mature embryos were subjected to experimental treatments to detect optimum doses of mutagenesis and to estimate polymorphism and genomic instability. Primarily, 50% reduction in number of regenerated plants as compared to the control (LD50) was adopted as the optimum dose. Based on LD50 criterion, the optimum value was achieved at 1 h duration of 4 mM SA concentration.
Afterwards, inter-primer binding site markers were applied to investigate polymorphism and genomic instability in the regenerated plants.
Conclusions
Present findings revealed that efficiency of chemical mutagenesis could be improved through the use of molecular technology and such mutations may assist plant breeders in developing high-yield cultivars.
Similar content being viewed by others
References
Olaolorun BM, Shimelis H, Laing M, Mathew I (2021) Development of wheat (Triticum aestivum L.) populations for drought tolerance and improved biomass allocation through ethyl methanesulfonate mutagenesis. Front Agron. https://doi.org/10.3389/fagro.2021.655820
Wang TL, Uauy C, Robson F, Till B (2012) TILLING in extremis. Plant Biotechnol J 10(7):761–772. https://doi.org/10.1111/j.1467-7652.2012.00708.x
Yunus MF, Abd Aziz M, Kadir MA, Daud SK, Rashid AA (2013) In vitro mutagenesis of Etlingera elatior (Jack) and early detection of mutation using RAPD markers. Turk J Biol 37(6):716–725. https://doi.org/10.3906/biy-1303-19
Ness RW, Morgan AD, Colegrave N, Keightley PD (2012) Estimate of the spontaneous mutation rate in Chlamydomonas reinhardtii. Genetics 192(4):1447–1454. https://doi.org/10.1534/genetics.112.145078
Kozgar MI, Kozgar I (2014) Mutation breeding in chickpea. De Gruyter Open Poland. https://doi.org/10.2478/9788376560717
Khan S, Al-Qurainy F, Anwar F (2009) Sodium azide: a chemical mutagen for enhancement of agronomic traits of crop plants. Environ We Int J Sci Tech 4:1–21
Mullins E, Bresson JL, Dalmay T, Dewhurst IC, Epstein MM, Firbank LG, Guerche P, Hejatko J, Moreno FJ (2021) In vivo and in vitro random mutagenesis techniques in plants. EFSA J 19(11):e06611. https://doi.org/10.2903/j.efsa.2021.6611
Lu G, Zhang X, Zou Y, Zou Q, Xiang X, Cao J (2007) Effect of radiation on regeneration of Chinese narcissus and analysis of genetic variation with AFLP and RAPD markers. Plant Cell Tissue Organ Cult 88(3):319–327. https://doi.org/10.1007/s11240-006-9189-9
Serrat X, Esteban R, Guibourt N, Moysset L, Nogués S, Lalanne E (2014) EMS mutagenesis in mature seed-derived rice calli as a new method for rapidly obtaining TILLING mutant populations. Plant Methods 10(1):5. https://doi.org/10.1186/1746-4811-10-5
Szarejko I, Forster B (2007) Doubled haploidy and induced mutation. Euphytica 158(3):359–370. https://doi.org/10.1007/s10681-006-9241-1
Ahloowalia B, Maluszynski M (2001) Induced mutations— a new paradigm in plant breeding. Euphytica 118(2):167–173. https://doi.org/10.1023/A:1004162323428
Das A, Gosal S, Sidhu J, Dhaliwal H (2000) Induction of mutations for heat tolerance in potato by using in vitro culture and radiation. Euphytica 114(3):205–209
El-Sayed O, Rizkalla A, Sabri S (2007) In vitro mutagenesis for genetic improvement of salinity tolerance in wheat. J Agric Biol Sci 4(5):377–383
Predieri S (2001) Mutation induction and tissue culture in improving fruits. Plant Cell Tissue Organ Cult 64(2–3):185–210
Saif-Ur-Rasheed M, Asad S, Zafar Y, Waheed R (2001) Use of radiation and in vitro techniques for development of salt tolerant mutants in sugarcane and potato, pp 61–74
Saleem M, Mukhtar Z, Cheema A, Atta B (2005) Induced mutation and in vitro techniques as a method to induce salt tolerance in Basmati rice (Oryza sauva L.). Int J Environ Sci Technol 2(2):141–145
Datta SK (2012) Success story of induced mutagenesis for development of new ornamental varieties. Bioremediat Biodivers Bioavailab 6(1):15–26
Hosseinpour A, Aydin M, Haliloglu K (2019) Plant regeneration system in recalcitrant rye (Secale cereale L.). Biologia 75:1017–1028. https://doi.org/10.2478/s11756-019-00395-9
Govindaraju S, Arulselvi PI (2018) Effect of cytokinin combined elicitors (l-phenylalanine, salicylic acid and chitosan) on in vitro propagation, secondary metabolites and molecular characterization of medicinal herb–Coleus aromaticus Benth (L). J Saudi Soc Agric Sci 17(4):435–444. https://doi.org/10.1016/j.jssas.2016.11.001
Erturk FA, Agar G, Arslan E, Nardemir G (2015) Analysis of genetic and epigenetic effects of maize seeds in response to heavy metal (Zn) stress. Environ Sci Pollut Res 22(13):10291–10297. https://doi.org/10.1007/s11356-014-3886-4
Aydin M, Hosseinpour A, Halİloğlu K, Tosun M (2016) Effect of polyamines on somatic embryogenesis via mature embryo in wheat. Turk J Biol 40(6):1178–1184. https://doi.org/10.3906/biy-1601-21
Zeinalzadehtabrizi H, Hosseinpour A, Aydin M, Haliloglu K (2015) A modified genomic DNA extraction method from leaves of sunflower for PCR based analyzes. J Biol Environ Sci 7:222–225
Kalendar R, Antonius K, Smýkal P, Schulman AH (2010) iPBS: a universal method for DNA fingerprinting and retrotransposon isolation. Theoret Appl Genet 121(8):1419–1430. https://doi.org/10.1007/s00122-010-1398-2
Hosseinpour A, Ilhan E, Özkan G, Öztürk Hİ, Haliloglu K, Cinisli KT (2021) Plant growth-promoting bacteria (PGPBs) and copper (II) oxide (CuO) nanoparticle ameliorates DNA damage and DNA Methylation in wheat (Triticum aestivum L.) exposed to NaCl stress. J Plant Biochem Biotechnol. https://doi.org/10.1007/s13562-021-00713-w
Taheri S, Abdullah TL, Jain SM, Sahebi M, Azizi P (2017) TILLING, high-resolution melting (HRM), and next-generation sequencing (NGS) techniques in plant mutation breeding. Mol Breed 37(3):40. https://doi.org/10.1007/s11032-017-0643-7
Sikora P, Chawade A, Larsson M, Olsson J, Olsson O (2011) Mutagenesis as a tool in plant genetics, functional genomics, and breeding. Int J Plant Genomics. https://doi.org/10.1155/2011/314829
Mohan Jain S, Suprasanna P (2011) Induced mutations for enhancing nutrition and food production. Gene Conserve, 10(41)
Suprasanna P, Jain SM, Ochatt S, Kulkarni V, Predieri S (2012) Applications of in vitro techniques in mutation breeding of vegetatively propagated crops. Plant mutation breeding and biotechnology. CABI, Wallingford, pp 371–385
Suprasanna P, Mirajkar S, Patade V, Jain SM (2014) Induced mutagenesis for improving plant abiotic stress tolerance. Mutagenesis: exploring genetic diversity of crops. Wageningen Academic Publishers, Wageningen, pp 345–376. https://doi.org/10.3920/978-90-8686-796-7_17
Riaz A, Gul A (2015) Plant mutagenesis and crop improvement. Agric Ecosyst Environ Issues. Springer, Cham, pp 181–209. https://doi.org/10.1007/978-3-319-23162-4_8
Foolad, M (2007) Advances in molecular breeding toward drought and salt tolerant crops. In: Jenks MA, Hasegawa PM, Jain SM (eds). Springer, New York, pp 1–32
Spencer-Lopes M, Forster BP, Jankuloski L (2018) Manual on mutation breeding. Food and Agriculture Organization of the United Nations (FAO), Paris
Kannan B, Davila-Olivas NH, Lomba P, Altpeter F (2015) In vitro chemical mutagenesis improves the turf quality of bahiagrass. Plant Cell, Tissue Organ Cult. (PCTOC) 120(2):551–561. https://doi.org/10.1007/s11240-014-0621-2
Srivastava RK, Sandhu AS, Gosal SS (2001) Effect of in vitro mutagenesis on plant regeneration in Citrus aurantifolia S. -Mutat Breed Newslett 45:48–50
Ali A, Naz S, Alam SS, Iqbal J (2007) In vitro induced mutation for screening of red rot (Colletotrichum falcatum) resistance in sugarcane (Saccharum officinarum). Pak J Bot 39(6):1979–1994
Bhagwat B, Duncan E (1998) Mutation breeding of banana cv. Highgate (Musa spp., AAA Group) for tolerance to Fusarium oxysporum f. sp. cubense using chemical mutagens. Sci Hortic 73(1):11–22. https://doi.org/10.1016/S0304-4238(97)00141-6
Castillo A, Cistue L, Valles M, Sanz J, Romagosa I, Molina-Cano J (2001) Efficient production of androgenic doubled-haploid mutants in barley by the application of sodium azide to anther and microspore cultures. Plant Cell Rep 20(2):105–111
He J, Hu Y, Li W-C, Fu F-L (2009) Drought tolerant mutant induced by gamma-ray and sodium azide from maize calli. Maize Genet Coop News Lett 83:53–55
Jeng T, Tseng T, Wang C, Chen C, Sung J (2003) Starch biosynthesizing enzymes in developing grains of rice cultivar Tainung 67 and its sodium azide-induced rice mutant. Field Crops Res 84(3):261–269. https://doi.org/10.1016/S0378-4290(03)00094-7
Jeng TL, Tseng TH, Wang CS, Chen CL, Sung JM (2006) Yield and grain uniformity in contrasting rice genotypes suitable for different growth environments. Field Crops Res 99(1):59–66. https://doi.org/10.1016/j.fcr.2006.03.005
Mondal S, Badigannavar AM, Kale DM, Murty GSS (2007) Induction of genetic variability in a disease resistant groundnut breeding line. Newsletter, Founders Day Special (285)
Nakata Y, Ueno M, Kihara J, Ichii M, Taketa S, Arase S (2008) Rice blast disease and susceptibility to pests in a silicon uptake-deficient mutant lsi1 of rice. Crop Prot 27(3–5):865–868. https://doi.org/10.1016/j.cropro.2007.08.016
Oliver R, Yang C, Hu G, Raboy V, Zhang M (2009) Identification of PCR-based DNA markers flanking three low phytic acid mutant loci in barley. J Plant Breed Crop Sci 1(4):087–093. https://doi.org/10.5897/JPBCS.9000081
Rascio A, Russo M, Mazzucco L, Platani C, Nicastro G, Di Fonzo N (2001) Enhanced osmotolerance of a wheat mutant selected for potassium accumulation. Plant Sci 160(3):441–448. https://doi.org/10.1016/S0168-9452(00)00404-0
Škorić D, Jocić S, Sakač Z, Lečić N (2008) Genetic possibilities for altering sunflower oil quality to obtain novel oils. Can J Physiol Pharmacol 86(4):215–221. https://doi.org/10.1139/Y08-008
Suzuki Y, Sano Y, Ise K, Matsukura U, Aoki N, Sato H (2008) A rice mutant with enhanced amylose content in endosperm without affecting amylopectin structure. Breed Sci 58(3):209–215. https://doi.org/10.1270/jsbbs.58.209
Venegas-Calerón M, Martínez-Force E, Garcés R (2008) Lipid characterization of a wrinkled sunflower mutant. Phytochemistry 69(3):684–691. https://doi.org/10.1016/j.phytochem.2007.09.026
Pande S, Khetmalas M (2012) Biological effect of sodium azide and colchicine on seed germination and callus induction in Stevia rebaudiana. Asian J Exp Biol Sci 3(1):93–98
Jain SM (2005) Major mutation-assisted plant breeding programs supported by FAO/IAEA. Plant Cell Tissue Organ Cult 82(1):113–123
Viana VE, Pegoraro C, Busanello C, de Oliveira AC (2019) Mutagenesis in rice: the basis for breeding a new super plant. Front Plant Sci. https://doi.org/10.3389/fpls.2019.01326
Funding
In vitro mutagenesis section in this study presents partial outcomes of Ph.D. thesis of Aras Turkoglu, supported by the Scientific and Technological Research Council of Turkey (TUBITAK, Project no. TOVAG 113O940).
Author information
Authors and Affiliations
Contributions
Conceptualization of research (KH); Designing of the experiments (AT, MT, KH); Contribution of experimental materials (MT, KH); Execution of lab experiments and data collection (AT, KH); Analysis of data and interpretation (AT); Preparation of manuscript (AT, KH).
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Ethical approval
This manuscript does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
All authors reviewed and approved the final version for publication.
Consent to publish
All authors reviewed and approved the final version for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Türkoğlu, A., Tosun, M. & Haliloğlu, K. Mutagenic effects of sodium azide on in vitro mutagenesis, polymorphism and genomic instability in wheat (Triticum aestivum L.). Mol Biol Rep 49, 10165–10174 (2022). https://doi.org/10.1007/s11033-022-07896-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07896-y