Abstract
Background
Anther culture has become an important part of pepper breeding. Response to haploidy via androgenesis is highly genotype-specific. However, studies on the inheritance of response to anther culture are lacking. Therefore, the study aimed to determine the inheritance of androgenesis.
Methods and results
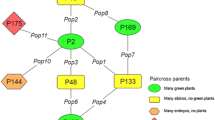
The plant material included crosses involving Capsicum annuum L. (253 A, and Inan3363) X C. chinense PI 159,236 populations. To estimate the heritability of the trait, two pepper lines with very high androgenesis responses were crossed with PI 159,236, a non-responsive accession. The androgenesis response was phenotyped using the parents, F1, F2, BC1P1, and BC1P2/P3 created through reciprocal crosses. Using variance components, the number of genes controlling the trait, broad (H2 = 0.97), and narrow sense heritabilities (h2 = 0.20), genetic variance (VG=113.8), the additive (VA=23.9), and the dominance gene variances (VD = 89.9), as well as the environmental variance (VE = 3.6) were calculated. Additive and dominant gene effects were 21% and 79%, respectively. Results derived from two different populations showed that the number of genes controlling the trait was between 1.96 and 2.46, and H2 = 0.96–0.97, h2 = 0.20–0.65, VG=91.8-113.8, VA, = 23.9–62.7, VD = 29.1–89.9, and VE=3.5–3.6 were calculated. The X2 analysis indicated that the most suitable one is the 9: 3: 4 epistatic genetic model (X2 = 2.13, P = 0.343, N = 155).
Conclusions
Results obtained from two different populations indicate the existence of a few major genes for response to androgenesis in pepper. Elucidating the inheritance of androgenesis is expected to pave the way for tagging the gene(s) in the pepper genome.


Similar content being viewed by others
Data availability
The datasets used for the current study are available from the corresponding author on reasonable request.
References
FAOSTAT (2020) Food and Agriculture Organization of the United Nations. http://www.fao.org/faostat/en/ (Accessed 01.14.2022)
Shmykova NA, Pyshnaya ON, Shumilina DV, Dzhos EA (2014) Morphological characteristics of doubled haploid plants of pepper produced using microspore/anther in vitro culture of the interspecies hybrids of Capsicum annum L. and C. chinense Jacq. Russ Agricult Sci 40(6):417–421. https://doi.org/10.3103/S1068367414060202
Atanassov A, Zagorska N, Boyadjiev P, Djilianov D (1995) In vitro production of haploid plants. World J Microbiol Biotechnol 11:400–408. https://doi.org/10.1007/BF00364615
Lefebvre V, Palloix A (1996) Both epistatic and additive effects of QTL are involved in polygenic induced resistance to disease: a case study, the interaction pepper-Phytophthora capsici Leonian. Theor Appl Genet 93:503–511. https://doi.org/10.1007/BF00417941
Steinitz B, Wolf D, Matzevitch-Josef T, Zelcer A (1999) Regeneration in vitro and genetic transformation of pepper (Capsicum spp.): the current state of the art. Capsicum Eggplant Newsl 18:9–15
Zagorska NA, Dimitrov BD, Ilieva I, Gadeva P (2002) In vitro induction of androgenesis and gynogenesis in Rubus caesius L. and Rubus idaeus L. Genet Breed 31:3–13
Andres MSA, Garcel CA, Esteban CJ, Peiro AJL, Palazon C, Luis Arteaga M, Gil Ortega R (2004) Application of anther culture and molecular markers to a pepper breeding program for diseases resistance. Capsicum Eggplant Newsl 23:105–108
Pauk J, Lantos C, Somogyi G, Vagi P, Abraham TZ, Gemes JA, Mihaly R, Kristof Z, Somogyi N, Timar Z (2010) Tradition, quality and biotechnology in Hungarian spice pepper (Capsicum annuum L.) breeding. Acta Agron Hung 58(3):259–266. https://doi.org/10.1556/AAgr.58.2010.3.8
Jha K, Choudhary PK, Agarwal A (2021) Doubled haploid production in Capsicum annuum L. using anther culture. Rev Plant Arc 21:168–173. https://doi.org/10.51470/PLANTARCHIVES.2021.v21.S1.031
Germana MA (2011) Gametic embryogenesis and haploid technology as valuable support to plant breeding. Plant Cell Rep 30:839–857. https://doi.org/10.1007/s00299-011-1061-7
Parra-Vega V, Seguí-Simarro JM Anther Culture in Pepper (Capsicum annuum L.) (Germana M(2016) M. Lambardi editors). In Vitro Embryogenesis in Higher Plants. Methods in Molecular Biology, Vol 1359. New York
Irikova TP, Kintzios S, Grozeva S, Rodeva V (2016) Pepper (Capsicum annuum L.) anther culture: fundamental research and practical applications. Turkish J Biology 40:719–726. doi:https://doi.org/10.3906/biy-1506-79
De Dumas R, Chambonnet D, Sibi M (1981) Culture in vitro d’antheres de piment (Capsicum annuum L.): amelioration des taux d’obtention de plantes chez differents genotypes par de traitments a + 35°C. Agronomie 11:859
Niazian M, Shariatpanahi ME (2020) In vitro-based doubled haploid production: recent improvements Euphytica. 216:69. https://doi.org/10.1007/s10681-020-02609-7. 5
Çömlekçioğlu N, Büyükalaca S, Abak K(2001) Effect of silver nitrate on haploid embryo induction by anther culture in pepper (Capsicum annuum). XIth EUCARPIA Meeting on Genetics and Breeding of Capsicum & Eggplant, Antalya-Turkey 133–136
Ciner OD, Tıpırdamaz R(2002) The effect of cold treatment and charcoal on the in vitro androgenesis of pepper (Capsicum annuum L.). Turk J Bot, 26(2002): 131–139
Buyukalaca S, Comlekcioglu N, Abak K, Ekbic N, Kilic N (2004) Effect of silver nitrate and donor plant growing conditions on the production of pepper (Capsicum annuum L.) haploid embryos via anther culture. Europ J Hort Sci 69(5):206–209E
Ercan N, Sensoy AF, Sensoy AS (2006) Influence of growing season and donor plant age on anther culture response of some pepper cultivars (Capsicum annuum L.). Scientia Hort 110:16–20. https://doi.org/10.1016/j.scienta.2006.06.007
Ata A(2011) Effect of season on pepper (Capsicum annuum L.) anther culture and microspore development. MSc Thesis, Department of Horticulture Institute of Natural and Applied Sciences, University of Cukurova
Taşkin H, Büyükalaca S, Keleş D, Ekbiç E (2011) Induction of microspore-derived embryos by anther culture in selected pepper genotypes. Afr J Biotechnol 10(75):17116–17121. https://doi.org/10.5897/AJB11.2023
Supena EDJ (2021) An Efficient Anther Culture on Double-Layered Media to Produce Doubled Haploid Plants of Pepper (Capsicum annuum). Doubled Haploid Technology, pp 267–278
Alremi F, Taşkın H, Sönmez K, Büyükalaca S, Ellialtioğlu Ş (2014) Effect of Genotype and Nutrient Medium on Anther Culture of Pepper (Capsicum annuum L.). Turkish J Agricultural Nat Sci 1(2):108–116
Olszewska D, Jedrzejczyk I, Nowaczyk P, Sendel S, Gaczkowska B (2015) In Vitro Colchicine Treatment of Anther-Derived Pepper Haploids. Bulgarian J Agricultural Sci 21(4):806–810
Arı E, Bedir H, Yıldırım S, Yıldırım T (2016) Androgenic responses of 64 ornamental pepper (Capsicum annuum L.) genotypes to shed-microspore culture in the autumn season. Turk J Biol 40:706–717. https://doi.org/10.3906/biy-1505-41
Ata A(2019) Investigation of The Possibilities of Creating Fertile Solanum melongena × Solanum torvum Hybrid Populations in Eggplant and Dihaploidization Through Androgenesis in Eggplant Rootstocks. Ph.D. Thesis, Department of Horticulture Institute of Natural and Applied Sciences, University of Cukurova
Grozeva S, Nankar AN (2020) Effect of incubation period and culture medium on pepper anther culture. Indian J Biotechnol 19:53–59
Rudolf K, Bohanec B, Hansen M (1999) Microspore culture of white cabbage, Brassica oleracea var. capitata L.: Genetic improvement of non-responsive cultivars and effect of genome doubling agents. Plant Breeding 118:237–241. https://doi.org/10.1046/j.1439-0523.1999.118003237.x
Afele JC, Kannenberg LW (1990) Genetic studies of corn (Zea mays L.) anther culture response. Theor Appl Genet 80:459–464. https://doi.org/10.1007/BF00226745
Agache S, Bachelier B, Buyser J, Henry Y, Snape J (1989) Genetic analysis of anther culture response in wheat using aneuploid chromosome substitution and translocation lines. Theor Appl Genet 77:7–11. https://doi.org/10.1007/BF00292308
Foroughi-Wehr B, Friedt W, Wenzel G (1982) On the genetic improvement of androgenetic haploid formation in Hordeum vulgate L. Theor Appl Genet 62:233–239. https://doi.org/10.1007/BF00276246
Raquin C (1982) Genetic control of embryo production and embryo quality in anther culture of Petunia. Theor Appl Genet 63:151–154. https://doi.org/10.1007/BF00303698
Miah MAA, Earle ED, Khush GS (1985) Inheritance of callus formation ability in anther cultures of rice, Oryza sativa L. Theor Appl Genet 70:113–116. https://doi.org/10.1007/BF00275308
Rakoczy-Trojanowska M, Malepszy S (1995) Genetic factors influencing the regeneration ability of rye (Secale cereale L.) II. Immature embryos. Euphytica 83:233–239. https://doi.org/10.1007/BF01678135
Begheyn R, Roulund N, Vangsgaard K, Kopecký D, Studer B(2017) Inheritance patterns of the response to in vitro doubled haploid induction in perennial ryegrass (Lolium perenne L.). Plant Cell Tiss Organ Cult (2017) 130:667–679 DOI https://doi.org/10.1007/s11240-017-1255-y
Smykal P (2000) Pollen Embryogenesis–The Stress Mediated Switch from Gametophytic to Sporophytic Development. Current Status and Future Prospects. Biol Plant 43:481–489. https://doi.org/10.1023/A:1002835330799
Ata A, Keleş D, Taşkın H, Büyükalaca S (2019) Effects of season, genotype, and nutrient medium on pepper anther culture and microspore development. Turk J Agric For 43:123–137. https://doi.org/10.3906/tar-1802-35
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiologia Plant 15:473–497
Wright S (1968) Evolution and the Genetics of Populations, vol 1. University of Chicago Press, Chicago, US
Allard RW (1960) Principles of Plant Breeding. John Wiley & Sons, New York
Powell W (1998) Diallel analysis of barley anther culture response. Genome 30:152–157. https://doi.org/10.1139/g88-026
Koleva-Gudeva LR, Spasenoski M, Trajkova F (2007) Somatic embryogenesis in pepper anther culture: The effect of incubation treatments and different media. Sci Hortic 111:114–119. https://doi.org/10.1016/j.scienta.2006.10.013
Nowaczyk P, Nowaczyk L, Olszewska D, Krupska A (2009) Androgenic response of genotypes selected from Capsicum annuum L. x C. chinense Jacq. Hybrids Acta Physiol Plant 31:877–879
Bagheri N, Jeloder NB (2008) Combining ability and heritability of callus induction and green plant regeneration in rice anther culture. Biotechnology 7(2):287–292. https://doi.org/10.3923/biotech.2008.287.292
Petolino JF, Thompson SA (1987) Genetic analysis of anther culture response in maize. Theor Appl Genet 76:157–159. https://doi.org/10.1007/BF00289982
He P, Chen Y, Shen Y, Lu C, Zhu L (1997) Inheritance of callus induction ability in rice anther culture. Chin Sci Bull 42(14):1209–1211. https://doi.org/10.1007/BF02882850
Yamagishi M, Otani M, Higashi M, Fukuta Y, Fukui K, Yano M, Shimada T (1998) Chromosomal regions controlling anther culturability in rice (Oryza sativa L.). Euphytica 103:227–234
Kwon YS, Kim KM, Eun MY, Sohn SK (2002) QTL mapping and associated marker selection for the efficacy of green plant regeneration in anther culture. Plant Breed 121:10–16. https://doi.org/10.1046/j.1439-0523.2002.00664.x
Singsit C, Veilleux RE (1989) Intra- and interspecific transmission of androgenetic competence in diploid potato species. Euphytica 43:105–112. https://doi.org/10.1007/BF00037902
Veronneau H, Lavoie G, Cappadocia M (1992) Genetic analysis of anther and leaf disc culture in two clones of Solanum chacoense Bitt. and their reciprocal hybrids. Plant Cell Tiss Org 30:199–209. https://doi.org/10.1007/BF00040022
Begheyn RF, Yates SA, Sykes T, Studer B (2018) Genetic loci governing androgenic capacity in perennial ryegrass (Lolium perenne L.). G3: Genes|Genomes|Genetics Early Online. https://doi.org/10.1534/g3.117.300550
Funding
This study was supported by the Republic of Turkey Ministry of Agriculture and Forestry General Directorate of Agricultural Research and Policies (TAGEM/BBAD/12/A09/P01/03) and Cukurova University Scientific Research Projects Coordinating Office (FDK-2016-2414) financially.
Author information
Authors and Affiliations
Contributions
Nihal Denli was responsible for writing the manuscript and for conducting and collecting data and interpretation of data. Atilla Ata was responsible for conducting and collecting data. Davut Keleş was responsible for the interpretation of data. Nedim Mutlu was responsible for the interpretation of data and for writing the manuscript. Hatıra Taşkın was responsible for the interpretation of data and for writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant fnancial or non-fnancial interests to disclose.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Denli, N., Ata, A., Keleş, D. et al. Inheritance of androgenesis response in pepper. Mol Biol Rep 49, 11601–11609 (2022). https://doi.org/10.1007/s11033-022-07876-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07876-2