Abstract
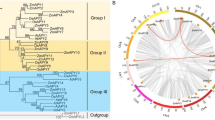
Heat shock protein 70 (HSP70) proteins play a crucial role in mitigating the detrimental effects of abiotic stresses in plants. In the present study, 21 full length non-redundant SlHSP70 genes were detected and characterized in tomato (Solanum lycopersicum L.). The SlHSP70 genes were classified into four groups based on phylogenetic analysis. Similarities were observed in gene features and motif structures of SlHSP70s belonging to the same group. SlHSP70 genes were unevenly and unequally mapped on 11 chromosomes. Segmental and tandem duplication are the main events that have contributed to the expansion of the SlHSP70 genes. A large number of groups and sub-groups were generated during comparative analysis of HSP70 genes in multiple plant species including tomato. These findings indicated a common ancestor which created diverse sub-groups prior to a mono-dicot split. The selection pressure on specific codons was identified through a maximum-likelihood approach and we found some important coding sites in the coding region of all groups. Diversifying positive selection was indirectly associated with evolutionary changes in SlHSP70 proteins and suggests that gene evolution modulated the tomato domestication event. In addition, expression analysis using RNA-seq revealed that 21 SlHSP70 genes were differentially expressed in response to drought and heat stress. SlHSP70-5 was down-regulated by heat treatment and up-regulated by drought stress. Furthermore, the expression of some of the duplicate genes was partially redundant, while others showed functional diversity. Our results indicate the diverse role of HSP70 gene family in S. lycopersicum under drought and heat stress conditions and open the gate for further investigation of HSP70 gene family functions, especially under drought and heat stress.





Similar content being viewed by others
References
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9(5):244–252. https://doi.org/10.1016/j.tplants.2004.03.006
Kotak S, Larkindale J, Lee U, von Koskull-Döring P, Vierling E, Scharf KD (2007) Complexity of the heat stress response in plants. Curr Opin Plant Biol 10(3):310–316. https://doi.org/10.1016/j.pbi.2007.04.011
Lata C, Yadav A, Prasad M (2011) Role of plant transcription factors in abiotic stress tolerance. Abiotic stress response in plants, vol 10. INTECH Open Access Publishers, London, pp 269–296
Waters ER (2013) The evolution, function, structure, and expression of the plant sHSPs. J Exp Bot 64(2):391–403. https://doi.org/10.1093/jxb/ers355
Datta K, Rahalkar K, Dinesh D (2017) Heat shock proteins (Hsp): classifications and its involvement in health and disease. J Pharma Care Health Syst 4(2):1–3
Renner T, Waters ER (2007) Comparative genomic analysis of the Hsp70s from five diverse photosynthetic eukaryotes. Cell Stress Chaperones 12(2):172
Ritossa F (1996) Discovery of the heat shock response. Cell Stress Chaperones 1(2):97–98. https://doi.org/10.1379/1466-1268(1996)001%3c0097:dothsr%3e2.3.co;2
Sung DY, Kaplan F, Guy CL (2001) Plant Hsp70 molecular chaperones: protein structure, gene family, expression and function. Physiol Plant 113(4):443–451
Kampinga HH, Craig EA (2010) The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 11(8):579–592
Mayer MP, Bukau B (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62(6):670–684. https://doi.org/10.1007/s00018-004-4464-6
Tomiczek B, Delewski W, Nierzwicki L, Stolarska M, Grochowina I, Schilke B, Dutkiewicz R, Uzarska MA, Ciesielski SJ, Czub J, Craig EA, Marszalek J (2020) Two-step mechanism of J-domain action in driving Hsp70 function. PLoS Comput Biol 16(6):e1007913. https://doi.org/10.1371/journal.pcbi.1007913
Kim JY, Barua S, Huang MY, Park J, Yenari MA, Lee JE (2020) Heat shock protein 70 (HSP70) induction: chaperonotherapy for neuroprotection after brain injury. Cells 9(9):2020. https://doi.org/10.3390/cells9092020
Zuiderweg ER, Hightower LE, Gestwicki JE (2017) The remarkable multivalency of the Hsp70 chaperones. Cell Stress Chaperones 22(2):173–189
Collins EJ, Bowyer C, Tsouza A, Chopra M (2022) Tomatoes: an extensive review of the associated health impacts of tomatoes and factors that can affect their cultivation. Biology (Basel) 11(2):239
Song B, Liu K, Gao Y, Zhao L, Fang H, Li Y, Pei L, Xu Y (2017) Lycopene and risk of cardiovascular diseases: a meta-analysis of observational studies. Mol Nutr Food Res 61(9):1601009
Niinemets Ü, Valladares F (2004) Photosynthetic acclimation to simultaneous and interacting environmental stresses along natural light gradients: optimality and constraints. Plant Biol 6(03):254–268
El Sappah A, Abbas M, Elrys AS, Yadav V, El-Sappah HH, Zhu Y, Huang Q, Yu W, Soaud SA, Xianming Z (2021) The Hsp70 gene family in Solanum lycopersicum; genome-wide identification and expression analysis under heavy metals stresses.
Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, Rokhsar DS (2012) Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res 40:D1178–D1186. https://doi.org/10.1093/nar/gkr944
Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A (2003) ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 31(13):3784–3788. https://doi.org/10.1093/nar/gkg563
Chen C, Chen H, He Y, Xia R (2018) TBtools, a toolkit for biologists integrating various biological data handling tools with a user-friendly interface. BioRxiv. https://doi.org/10.1101/289660
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874. https://doi.org/10.1093/molbev/msw054
Simpson MG (2010) Plant systematics. Phylogenetic systematics, 2nd edn. Academic Press, Cambridge
Bailey TL, Williams N, Misleh C, Li WW (2006) MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res 34:W369–W373. https://doi.org/10.1093/nar/gkl198
Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, Pang N, Forslund K, Ceric G, Clements J, Heger A, Holm L, Sonnhammer EL, Eddy SR, Bateman A, Finn RD (2012) The Pfam protein families database. Nucleic Acids Res 40:D290–D301. https://doi.org/10.1093/nar/gkr1065
Cannon SB, Mitra A, Baumgarten A, Young ND, May G (2004) The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol 4(1):10
Lee TH, Kim J, Robertson JS, Paterson AH (2017) Plant genome duplication database. Methods Mol Biol (Clifton, NJ) 1533:267–277. https://doi.org/10.1007/978-1-4939-6658-5_16
Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30(1):325–327. https://doi.org/10.1093/nar/30.1.325
Kosakovsky Pond S, Delport W, Muse SV, Scheffler K (2010) Correcting the bias of empirical frequency parameter estimators in codon models. PLoS ONE 5(7):e11230
Stanfel LE (1996) A new approach to clustering the amino acid. J Theor Biol 183(2):195–205
Delport W, Scheffler K, Botha G, Gravenor MB, Muse SV, Pond SLK (2010) CodonTest: modeling amino acid substitution preferences in coding sequences. PLoS Comput Biol 6(8):e1000885
Kosiol C, Holmes I, Goldman N (2007) An empirical codon model for protein sequence evolution. Mol Biol Evol 24(7):1464–1479
Davoudi M, Chen J, Lou Q (2022) Genome-wide identification and expression analysis of heat shock protein 70 (HSP70) gene family in pumpkin (Cucurbita moschata) rootstock under drought stress suggested the potential role of these chaperones in stress tolerance. Int J Mol Sci 23(3):1918
Zhang L, Zhao H-K, Dong Q-l, Zhang Y-Y, Wang Y-M, Li H-Y, Xing G-J, Li Q-Y, Dong Y-S (2015) Genome-wide analysis and expression profiling under heat and drought treatments of HSP70 gene family in soybean (Glycine max L.). Front Plant Sci 6:773
Zhang Y, Wang M, Chen J, Rong J, Ding M (2014) Genome-wide analysis of HSP70 superfamily in Gossypium raimondii and the expression of orthologs in Gossypium hirsutum. Yi Chuan = Hereditas 36(9):921–933
Cho EK, Choi YJ (2009) A nuclear-localized HSP70 confers thermoprotective activity and drought-stress tolerance on plants. Biotechnol Lett 31(4):597–606
Kose S, Furuta M, Imamoto N (2012) Hikeshi, a nuclear import carrier for Hsp70s, protects cells from heat shock-induced nuclear damage. Cell 149(3):578–589
Jung K-H, Gho H-J, Nguyen MX, Kim S-R, An G (2013) Genome-wide expression analysis of HSP70 family genes in rice and identification of a cytosolic HSP70 gene highly induced under heat stress. Funct Integr Genomics 13(3):391–402
Lin B-L, Wang J-S, Liu H-C, Chen R-W, Meyer Y, Barakat A, Delseny M (2001) Genomic analysis of the Hsp70 superfamily in Arabidopsis thaliana. Cell Stress Chaperones 6(3):201
Sarkar NK, Kundnani P, Grover A (2013) Functional analysis of Hsp70 superfamily proteins of rice (Oryza sativa). Cell Stress Chaperones 18(4):427–437
Lespinet O, Wolf YI, Koonin EV, Aravind L (2002) The role of lineage-specific gene family expansion in the evolution of eukaryotes. Genome Res 12(7):1048–1059
Sémon M, Wolfe KH (2007) Consequences of genome duplication. Curr Opin Genet Dev 17(6):505–512
Ahmad MZ, Sana A, Jamil A, Nasir JA, Ahmed S, HameedAbdullah MU (2019) A genome-wide approach to the comprehensive analysis of GASA gene family in Glycine max. Plant Mol Biol 100(6):607–620
Blanc G, Wolfe K (2004) Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell Online 16(7):1667–1678
Ha M, Kim E-D, Chen ZJ (2009) Duplicate genes increase expression diversity in closely related species and allopolyploids. Proc Natl Acad Sci USA 106(7):2295–2300
Force A, Lynch M, Pickett FB, Amores A, Yan Y-l, Postlethwait J (1999) Preservation of duplicate genes by complementary, degenerative mutations. Genetics 151(4):1531–1545
Fetterman CD, Rannala B, Walter MA (2008) Identification and analysis of evolutionary selection pressures acting at the molecular level in five forkhead subfamilies. BMC Evol Biol 8(1):261
Marini NJ, Thomas PD, Rine J (2010) The use of orthologous sequences to predict the impact of amino acid substitutions on protein function. PLoS Genet 6(5):e1000968
Czarnecka E, Key J, Gurley WB (1989) Regulatory domains of the Gmhsp17.5-E heat shock promoter of soybean. Mol Cell Biol 9(8):3457–3463
Yamaguchi-Shinozaki K, Shinozaki K (1994) A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6(2):251–264
Kurepa J, Wang S, Li Y, Smalle J (2009) Proteasome regulation, plant growth and stress tolerance. Plant Signal Behav 4(10):924–927
Acknowledgements
The authors thanks the whole team participated in this research work and present it in front of scientific community.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
MZA and ZS designed the research. MZA, ZS, AU, AK extracted the data. MZA, ZS and AU analysed and wrote the manuscript. MZA, BA and SA arranged and revised the manuscript. All the contributors read and approved for submission.
Corresponding author
Ethics declarations
Conflict of interest
All authors did not have any conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ahmad, M.Z., Shah, Z., Ullah, A. et al. Genome wide and evolutionary analysis of heat shock protein 70 proteins in tomato and their role in response to heat and drought stress. Mol Biol Rep 49, 11229–11241 (2022). https://doi.org/10.1007/s11033-022-07734-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07734-1