Abstract
Background
Hyperpigmentation, which causes excessive melanin synthesis and accumulation, is an important issue in the cosmetic industry. Since compounds developed against hyperpigmentation often come with side effects such as skin irritation and contact dermatitis, new studies focus on the use of natural agents that have no side effects.
Methods and Results
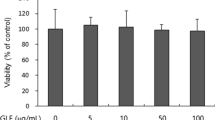
In this study, it was found that the effects of soybean cell culture extract (SCE) on alpha-melanocyte-stimulating hormone (α-MSH) induced melanogenesis in B16-F10 murine melanoma cells. The cells were incubated with SCE for 48 h after treatment with α‑MSH for 24 h to analysis the melanin content, cellular tyrosinase activity, and gene and protein expression. SCE at 1 mg/mL decreased melanin content and tyrosinase activity by 34% and 24%, respectively, compared to the α-MSH-treated group, which did not decrease cell viability. In addition, SCE (1 mg/mL) downregulated the expression of tyrosinase (TYR), tyrosinase-related protein (TRP)-1, tyrosinase-related protein (TRP)-2, and microphthalmia-associated transcription factor (MITF) genes 1.5-, 1.5-, 2-, and 2-fold, respectively. Furthermore, SCE inhibited the expression of TYR, TRP1, and TRP2 by decreasing the expression of MITF, as shown in a western blot assay.
Conclusions
This study suggests that SCE reveals dose-dependent inhibition of melanin synthesis through the suppression of tyrosinase activity as well as molecular levels of TYR, TRP1, TRP2, and MITF in B16-F10 murine melanoma cells. Therefore, SCE has the potential to be an effective and natural skin-whitening agent for application in the cosmetic industry.





Similar content being viewed by others
References
Seiberg M, Paine C, Sharlow E (2000) Inhibition of melanosome transfer results in skin lightening. J Invest Dermatol 115:162–167. https://doi.org/10.1046/j.1523-1747.2000.00035.x
Lee E, Cha J, HL (2019) Inonotus obliquus Extract as An Inhibitor of α-MSH-Induced Melanogenesis in B16-F10 Mouse Melanoma Cells. Cosmetics 6:1–9. https://doi.org/10.3390/cosmetics6010009
Pillaiyar T, Namasivayam V, Manickam M, Jung SH (2018) Inhibitors of Melanogenesis: An Updated Review. J Med Cem 61:7395–7418. https://pubs.acs.org/doi/abs/https://doi.org/10.1021/acs.jmedchem.7b00967
Zhou J, Shang J, Ping F, Zhao G (2012) Alcohol extract from Vernonia anthelmintica (L.) willd seed enhances melanin synthesis through activation of the p38 MAPK signaling pathway in B16F10 cells and primary melanocytes. J Ethnopharmacol 143:639–647. https://doi.org/10.1016/j.jep.2012.07.030
Kim A, Ma JY (2014) Anti-melanogenic activity of the novel herbal medicine, MA128, through inhibition of tyrosinase activity mediated by the p38 mitogen‐activated protein kinases and protein kinase signaling pathway in B16F10 cells. Pharmacognosy Magazine 10:462–471. https://dx.doi.org/10.4103%2F0973-1296.139774
Phacharapiyangkul N, Thirapanmethee K, Sangiamsuntor K, Panich U, Lee CH (2019) Chomnawang, M. T. Effect of Sucrier Banana Peel Extracts on Inhibition of Melanogenesis through the ERK Signaling Pathway. Int J Med Sci 16:602–606. https://dx.doi.org/10.7150%2Fijms.32137
Chung YC, Ko JH, Kang HK, Kim S, Kang CI, Lee JN, Park SM, Hyun CG (2018) Antimelanogenic Effects of Polygonum tinctorium Flower Extract from Traditional Jeju Fermentation via Upregulation of Extracellular Signal-Regulated Kinase and Protein Kinase B Activation. Int J Mol Sci 19:1–15. https://dx.doi.org/10.3390%2Fijms19102895
Georgiev V, Slavov A, Vasileva I, Pavlov A (2018) Plant cell culture as emerging technology for production of active cosmetic ingredients. Eng Life Sci 18:779–798. https://doi.org/10.1002/elsc.201800066
Goyal A, Sharma A, Kaur J, Kumari S, Garg M, Sindhu RK, Rahma MDH, Akhtar MF, Tagde P, Najda A et al (2022) Bioactive-Based Cosmeceuticals: An Update on Emerging Trends. Molecules 27:1–28. https://doi.org/10.3390/molecules27030828
Trehan S, Kohn BM, Beri K (2017) Plant stem cells in cosmetics: current trends and future directions. Future Sci OA 3:2056–5623. https://dx.doi.org/10.4155%2Ffsoa-2017-0026
Eibl R, Meier P, Stutz I, Schildberger D, Hühn T, Eibl D (2018) Plant cell culture technology in the cosmetics and food industries: current state and future trends. Appl Microbiol Biotechnol 102:8661–8675. https://doi.org/10.1007/s00253-018-9279-8
Dzialo M, Mierziak J, Korzun U, Preisner M, Szopa J, Kulma A (2016) The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int J Mol Sci 17:160–201. https://doi.org/10.3390/ijms17020160
Masum MN, Yamauchi K, Mitsunaga T (2019) Tyrosinase inhibitors from Natural and synthetic sources. Reviews in Agricultural Science 7:41–58. https://doi.org/10.1007/s00018-005-5054-y
Jung YS, Rha CH, Bail MY, Kim BNI DO (2020) A brief history and spectroscopic analysis of soy isoflavones. Food Sci Biotechnol 29:1605–1617. https://doi.org/10.1007/s10068-020-00815-6
Hu C, Wonga WT, Wua R, Lai WF (2019) Biochemistry and use of soybean isoflavones in functional food development. Crit Rev Food Sci Nutr 5:1–15. https://doi.org/10.1080/10408398.2019.1630598
Chen KI, Erh MH, Su NW, Liu WH, Chou CC, Cheng KC (2012) Soyfoods and soybean products: from traditional use to modern applications. Appl Microbiol Biotechnol 96:9–22. https://doi.org/10.1007/s00253-012-4330-7
Rani D, Vimolmangkang S (2022) Trends in the biotechnological production of isoflavonoids in plant cell suspension cultures. Phytochem. https://doi.org/10.1007/s11101-022-09811-6
Chang TS, Ding HY, Lin HC (2005) Identifying 6,7,4’-Trihydroxyisoflavone as a Potent Tyrosinase Inhibitor. Biosci Biotechnol Biochem 69:1999–2001. https://doi.org/10.1271/bbb.69.1999
Park JS, Kim DH, Lee JK, Lee JY, Kim DH, Kim HK, Lee HJ, Kim HC (2010) Natural ortho-dihydroxyisoflavone derivatives from aged Korean fermented soybean paste as potent tyrosinase and melanin formation inhibitors. Bioorg Med Chem Lett 20:1162–1164. https://doi.org/10.1016/j.bmcl.2009.12.021
Lee YS, Kim HK, Lee KJ, Jeon HW, Cui S, Lee YM, Moon BJ, Kim YH, Lee YS (2010) Inhibitory effect of glyceollin isolated from soybean against melanogenesis in B16 melanoma cells. BMB Rep 43:461–467. https://doi.org/10.5483/bmbrep.2010.43.7.461
Loizzo MR, Tundis R, Menichini F (2012) Natural and Synthetic Tyrosinase Inhibitors as Antibrowning Agents: An Update. Compr Rev Food Sci Food Saf 11:378–398. https://doi.org/10.1111/j.1541-4337.2012.00191.x
Wang BS, Juang LJ, Yang JJ, Chen LY, Tai HM, Huang MH (2012) Antioxidant and Antityrosinase Activity of Flemingia macrophylla and Glycine tomentella Roots. Evidence-Based Complement Altern Med 2012:1–7. https://doi.org/10.1155/2012/431081
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat Protoc 2:875–877. https://doi.org/10.1038/nprot.2007.102
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Jin KS, Lee JY, Hyun SK, Kim BW, Kwon HJ (2015) Juniperus chinensis and the functional compounds, cedrol and widdrol, ameliorate α-melanocyte stimulating hormone-induced melanin formation in B16F10 cells. Food Sci Biotechnol 24:611–618. https://doi.org/10.1007/s10068-015-0080-5
Livak KJ, Schmmittgen TD (2001) Analysis of relative gene expression data using real- time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Chan YY, Kim KH, Cheah SH (2011) Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. J Ethnopharmacol 137:1183–1188. https://doi.org/10.1016/j.jep.2011.07.050
Alam MB, Bajpai VK, Lee J, Zhao P, Byeon JH, Ra JS, Majumder R, Lee JS, Yoon JI, Rather IA, Park YH, Kim K, Na M, Lee SH (2017) Inhibiton of melanogenesis by jineol from Scolopendra subspinipes mutilans via MAP-Kinase mediated MITF downregulation and the proteasomal degradation and the proteasomal degradation of tyrosinase. Sci Rep 7:1–7. https://doi.org/10.1038/srep45858
Lv J, Yang Y, Jia B, Li S, Zhang X, Gao R (2021) The Inhibitory Effect of Curcumin Derivative J147 on Melanogenesis and Melanosome Transport by Facilitating ERK-Mediated MITF Degradation. Front Pharmacol 12:1–12. https://doi.org/10.3389/fphar.2021.783730
Shim E, Song E, Choi KS, Choi HI, Hwang J (2017) Inhibitory effect of Gastrodia elata Blume extract on alpha-melanocyte stimulating hormone-induced melanogenesis in murine B16F10 melanoma. Nutr Res Pract 11:173–179. https://doi.org/10.4162/nrp.2017.11.3.173
Seo GY, Ha Y, Park AH, Kwon OW, Kim YJ (2019) Leathesia difformis Extract Inhibits α-MSH-Induced Melanogenesis in B16F10 Cells via Down-Regulation of CREB Signaling Pathway. Int J Mol Sci 20:1–16. https://doi.org/10.3390/ijms20030536
Juliano CCA (2022) Spreading of Dangerous Skin-Lightening Products as a Result of Colourism: A Review. Appl Sci 12:1–15. https://doi.org/10.3390
Sansanelli S, Zanichelli D, Filippini A, Ferri M, Tassoni A (2014) Production of free and glycosylated isoflavones in vitro soybean (Glycine max L.) hypocotyl cell suspensions and comparison with industrial seed extracts. Plant Cell Tiss Organ Cult 119:301–311. https://doi.org/10.1007/s11240-014-0534-0
Chung H, Hogan S, Zhang L, Rainey K, Zhou K (2008) Characterization and Comparison of Antioxidant Properties and Bioactive Components of Virginia Soybeans. J Agric Food Chem 56:11515–11519. https://doi.org/10.1021/jf800468z
Slavin M, Cheng M, Luther M, Kenworthy W, Yu L (2009) Antioxidant properties and phenolic, isoflavone, tocopherol and carotenoid composition of Maryland-grown soybean lines with altered fatty acid profiles. Food Chem 114:20–27. https://doi.org/10.1021/jf902609n
Peiretti PG, Karamac M, Janiak M, Longato E, Meineri G, Amarowicz R, Gai F (2019) Phenolic Composition and Antioxidant Activities of Soybean (Glycine max (L.) Merr.) Plant during Growth Cycle. Agronomy 9:1–15. https://doi.org/10.3390/agronomy9030153
Je JG, Jiang Y, Heo JH, Li X, Jeon YJ, Ryu BM (2022) Mitigative Effects of PFF-A Isolated from Ecklonia cava on Pigmentation in a Zebrafish Model and Melanogenesis in B16F10 Cells. Mar Drugs 20:1–12. https://doi.org/10.3390/md20020123
Lee HJ, An S, Bae S, Lee JH (2022) Diarylpropionitrile inhibits melanogenesis via protein kinase A/cAMP-response element-binding protein/microphthalmia- associated transcription factor signaling pathway in α-MSH- stimulated B16F10 melanoma cells. Korean J Physiol Pharmacol 26:113–123. https://doi.org/10.4196/kjpp.2022.26.2.113
Lee JI, Seo JH, Ko ES, Cho SM2, Kang JR, Jeong JH, Jeong Y et al (2021) Inhibition of melanogenesis by Aster yomena callus pellet extract in melanoma cells and patients with skin pigmentation. Int J Med Sci 18:3299–3308. https://doi.org/10.7150/ijms.62530
Paine C, Sharlow E, Liebel F (2001) An alternative approach to depigmentation by soybean extracts via inhibition of the PAR-2 pathway. J Invest Dermatol 116:587–595. https://doi.org/10.1046/j.1523-1747.2001.01291.x
Hermanns JF, Petit L, Martalo O, Piérard-Franchimont C, Cauwenbergh G, Piérard GE (2000) Unraveling the Patterns of Subclinical Pheomelanin-Enriched Facial Hyperpigmentation: Effect of Depigmenting Agents. Dermatology 201:118–122. https://doi.org/10.1159/000018473
Qian W, Liu W, Zhu D, Cao Y, Tang A, Gong G, Su H (2020) Natural skin–whitening compounds for the treatment of melanogenesis (Review). Experimental and Therapeutic Medicine 20:73–185. https://dx.doi.org/10.3892%2Fetm.2020.8687
Acknowledgements
The authors would like to acknowledgements the contribution of ACTV Biotechnology in the conduct of the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of potential conflicts of interest
The authors declare that they have no competing interests.
Research involving human participants and/or animals
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bodurlar, Y., Caliskan, M. Inhibitory activity of soybean (Glycine max L. Merr.) Cell Culture Extract on tyrosinase activity and melanin formation in alpha-melanocyte stimulating Hormone-Induced B16-F10 melanoma cells. Mol Biol Rep 49, 7827–7836 (2022). https://doi.org/10.1007/s11033-022-07608-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07608-6