Abstract
Background
Chickpea (Cicer arietinum L.) is an important kernel legume in the world. To optimize the plant tissue culture some experiments such as direct regeneration, proliferation, rooting shoots and somatic embryogenesis were done.
Methods and results
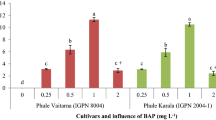
In experiments were used direct regeneration and proliferation, various levels of plant growth regulators NAA (0 and 1.0 mg/l), BAP (0, 1, 3 and 5 mg/l) and three explants’ types (epicotyl, cotyledon and embryonic axis). The results of both experiments showed that embryonic axis explant was better than other explants. The highest percentage was obtained in MS media containing 1 mg/l BAP and also 3 mg/l BAP and 0.1 mg/l NAA with an average of 72%. The highest average number of branches (4.66) was found in the proliferation of embryonic axis in MS medium containing 3 mg/l BAP. The highest rooting shoot (90%) was found in 1/2MS in B5 medium vitamins with 0.2 mg/l of IBA and 0.5 mg/l NAA. Somatic embryogenesis experiments were compared on the concentration gradient of 2,4-D in fine embryonic axis explants. The results displayed that the concentration gradient of 10 mg/l 2,4-D to 5 mg/l of 2,4-D and then to zero concentration showed the highest number of embryos.
Conclusion
The best environment for regeneration embryos was MS medium with 2.5 mg/l of 2,4-D concentration gradient to zero. In this study, the PCR reaction showed the presence of the β-glucuronidase (gus) marker gene in regenerated cotyledons for 20 min in all three strains studied.








Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BAP:
-
Benzylaminopurine
- BA:
-
Benzyl adenine
- NAA:
-
Naphthaleneacetic acid
- MS medium:
-
Murashige and Skoog medium
- PGRs:
-
Plant growth retardants
- IBA:
-
Indole-3-butyric acid
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- LB medium:
-
Lysogeny Broth medium
- DDW:
-
Deuterium-depleted water
- PCR:
-
Polymerase chain reaction
- ppm:
-
Part per million
- CTAB:
-
Cetyltrimethylammonium bromide
References
Tuna GS, Yucel G, Asciogul TK, Ates D, Esiyok D, Tanyolac MB, Tuna M (2020) Molecular cytogenetic characterization of common bean (Phaseolus vulgaris L.) accessions. Turk J Agric For 44:612–630. https://doi.org/10.3906/tar-1910-33
Ozturk HI, Dursun A, Hosseinpour A, Haliloglu K (2020) Genetic diversity of pinto and fresh bean (Phaseolus vulgaris L.) germplasm collected from Erzincan province of Turkey by inter-primer binding site (iPBS) retrotransposon markers. Turk J Agric For 44:417–427. https://doi.org/10.3906/tar-2002-9
Singh S, Singh I, Kapoor K, Gaur PM, Chaturvedi SK, Singh NP, Sandhu JS (2014) Chickpea. In: Singh M et al (eds) Broadening the Genetic Base of Grain Legumes. Springer, New Delhi
Gatti I, Guindón F, Bermejo C, Espósito A, Cointry E (2016) In vitro tissue culture in breeding programs of leguminous pulses: use and current status. Plant Cell Tissue Organ Cult 127:543–559
Bermejo C, Gatti I, Cointry E (2016) In vitro embryo culture to shorten the breeding cycle in lentil (Lens culinaris Medik). Plant Cell Tissue Organ Cult 127:585–590
Das A, Parida SK (2014) Advances in biotechnological applications in three important food legumes. Plant Biotechnol Rep 8:83–99
Leonetti P, Accotto GP, Hanafy MS, Pantaleo V (2018) Viruses and phytoparasitic nematodes of cicer arietinum L.: biotechnological approaches in interaction studies and for sustainable control. Front Plant Sci 9:319
Das Bhowmik SS, Cheng AY, Long H, Tan GZH, Hoang TML, Karbaschi MR, Williams B, Higgins TJV, Mundree SG (2019) Robust genetic transformation system to obtain non-chimeric transgenic Chickpea. Front Plant Sci 10:524
Yadava R, Mehrotra M, Singh AK, Niranjan A, Singh R, Sanyal I, Lehri A, Pande V, Amla DV (2017) Improvement in Agrobacterium-mediated transformation of chickpea (Cicer arietinum L) by the inhibition of polyphenolics released during wounding of cotyledonary node explants. Protoplasma 254(1):253–269
Kieber JJ, Schaller GC (2014) Cytokinins. Am Soc Plant Biol 12:e0168
Witjaksono T (1997) Development of protocols for Avocado tissue culture: somatic embryogenesis, protoplast culture, shoots proliferation and protoplast fusion. University of Florida, USA, Ph.D.Thesis
Von Arnold S, Sabala I, Bozhkov P, Dyachok J, Filonova L (2002) Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ Cult 69:233–249
Ibaraki Y, Kurata K (2001) Automation of somatic embryo production. Plant Cell Tissue Organ Cult 65:179–199
Wang X (1997) Somatic embryo induction and plant regeneration in American Ginseng (Panax quinquefolium L.). M.Sc. Thesis, University of Guelph, Canada
Tripathi L, Singh AK, Singh S, Singh R, Chaudhary S, Sanyal I, Amla DV (2013) Optimization of regeneration and Agrobacterium-mediated transformation of immature cotyledons of chickpea (Cicer arietinum L). Plant Cell Tissue Organ Cul 113(3):513
Roberson D, Cristiane L, Francine L, Henrique K, Marguerite Q (2005) Plant regeneration from cotyledonary explants of Eucalyptus camaldulensis. Sci Agri 62:406–412
Sanyal I, Singh AK, Kaushik M, Amla DV (2005) Agrobacterium mediated transformation of chickpea (Cicer arietinum L.) with Bacillus thuringiensis cry1Ac gene for resistance against pod borer insect Helicoverpa armigera. Plant Sci 168:1135–1146
Polisetty R, Paul V, Deveshvar JJ, Khetarpal S (1997) Multiple shoot induction by benzyladenine and complete plant regeneration from seed explants of chickpea (Cicer arietinum L.). Plant Cell Rep 16:565–571
Senthil G, Williamson B, Dinkins RD, Ramsay G (2004) An efficient transformation system for chickpea (Cicer arietinum L.). Plant Cell Rep 23:297–303
Krishnamurthy KV, Suhasini K, Sagare AP, Meixner M, Dekathen A, Pikardt T, Schieder O (2000) Agrobacterium mediated transformation of chickpea (Cicerarietinum L.) embryo axes. Plant Cell Rep 19:235–240
Shalini S, Batra P, Sindhu A, Chowdhury VK (2001) Multiple shoot induction and complete plant regeneration of chickpea (Cicerarietinum L.). Crop Res 21:308–311
Yousefiara M, Bagheri A, Moshtaghi N (2008) Optimizing regeneration condition in chickpea (Cicer arietinum L.). Pak J Biol Sci 11(7):1009–1014
Sharma K, Jayanand B, Sudarsanam G (2003) An efficient protocol for the regeneration of whole plants of chickpea (Cicer arietinum L.) by using axillary meristem explant derived from in vitro germinated seedings. In Vitro Cell Dev Biol Plant 39:171–179
Yusnita Y, Jamaludin J, Agustiansyah A, Hapsoro D (2018) A combination of IBA and NAA resulted in better rooting and shoot sprouting than single auxin on Malay Apple [Syzygium malaccense (L.) Merr. & Perry] Stem Cuttings. Agrivita J Agri Sci 40(1):80–90
Hartmann HT, Kester DE, Davies FT (1990) Plant Propagation: Principles and Practices. 5th ed., pp. 1–100. Prentice Hall International Editions, Englewood Cliffs, New Jersey, USA
Debnath M, Malik CP, Bisen PS (2006) Micropropagation: a tool for the production of high quality plant-based medicines. Curr Pharm Biotechnol 7:33–49
Nisha MC, Rajeshkumar S, Selvaraj T, Subramanian M (2009) A valued Indian medicinal plant Begonia malabarica Lam. Successful plant regeneration through various explants and field performance. Maejo Int J Sci Technol 3(02):261–268
Yuying L, Penghui D, Xianping Z (2010) Study on tissue culture of rieger begonia. Shaanxi Forestry Sci Technol 54:21–31
Quiroz-Figueroa FRR, Rojas Herrera RM, Galaz Avalos VM, Vargas L (2006) Embryo production through somatic embryogenesis can be used to study cell differentiation in plants. Plant Cell Tissue Organ Cult 86:285–301
Dita MA, Rispail N, Prats E, Rubiales D, Singh KB (2006) Biotechnology approaches to overcome biotic and abiotic stress constraints in legumes. Euphytica 147(1–2):1–24
Akbulut M, Yücel M, Öktem HA (2008) Analysis and optimization of DNA delivery into chickpea (Cicer arietinum L) seedlings by Agrobacterium tumefacience. Afr J Biotechnol 7(8):1
Varshney RK, Nayak SN, May GD, Jackson SA (2009) Nextgeneration sequencing technologies and their implications for crop genetics and breeding. Trends Biotechnol 27:522–530
Atif RM, Patat-Ochatt EM, Svabova L, Ondrej V, Klenoticova H, Jacas L, Griga M, Ochatt SJ (2013) Gene transfer in legumes. In: Luttge U, Beyschag W, Francis D, Cushman J (eds) Progress in botany 74. Springer-Verlag, Berlin, Heidelberg, pp 37–100
Batra P, Yadav NR, Sindhu A, Yadav RC, Chowdhury VK, Chowdhury JB (2002) Efcient protocol for in vitro direct plant regeneration in chickpea Cicer arietinum L. Indian J Expl Biol 40:600–602
Sanyal I, Singh AK, Amla DV (2003) Agrobacterium tumefaciens mediated transformation of chickpea (Cicer arietinum L.) using mature embryogenic axis and cotyledonary nodes. Indian J Biotechnol 2:524–532
Bhattacharjee B, Mohan M, Nair S (2010) Transformation of chickpea: effect of genotype, explant, Agrobacterium-strain and composition of culture medium. Biol Plant 54(1):21–32
Yang ZN, Ingelbrecht IL, louzada E, Skaria M, Mirkov TE, (2000) Agrobacterium-mediated transformation of the commercially important grapefruit cultivar Rio Red (Citrus paradisi Macf.). Plant Cell Rep 19:1203–1211
Fathi A, Barak M, Damandan M, Amani F, Moradpour R, Khalilova I, Valizadeh M (2021) Neonatal Screening for Glucose-6-phosphate dehydrogenase deficiency in Ardabil Province, Iran, 2018–2019. Cell Mol Biomed Rep 1(1):1–6
Tourang M, Fang L, Zhong Y, Suthar R (2021) Association between human endogenous retrovirus K gene expression and breast cancer. Cell Mol Biomed Rep 1(1):7–13
Bilal I, Xie S, Elburki M, Aziziaram Z, Ahmed S, Jalal BS (2021) Cytotoxic effect of diferuloylmethane, a derivative of turmeric on different human glioblastoma cell lines. Cell Mol Biomed Rep 1(1):14–22
Aziziaram Z, Bilal I, Zhong Y, Mahmod A, Roshandel M (2021) Protective effects of curcumin against naproxen-induced mitochondrial dysfunction in rat kidney tissue. Cell Mol Biomed Rep 1(1):23–32
Ercisli MF, Lechun G, Azeez S, Hamasalih R, Song S, Aziziaram Z (2021) Relevance of genetic polymorphisms of the human cytochrome P450 3A4 in rivaroxaban-treated patients. Cell Mol Biomed Rep 1(1):33–41
Azeez S, Jafar S, Aziziaram Z, Fang L, Mawlood A, Ercisli M (2021) Insulin-producing cells from bone marrow stem cells versus injectable insulin for the treatment of rats with type I diabetes. Cell Mol Biomed Rep 1(1):42–51
Van Eenennaam AL, De Figueiredo SF, Trott JF, Zilberman D (2021) Genetic engineering of livestock: the opportunity cost of regulatory delay. Annu Rev Anim Biosci 9:453–478
Akin M, Eyduran E, Niedz RP, Reed BM (2017) Developing hazelnut tissue culture medium free of ion confounding. Plant Cell Tissue Organ Cult 130(3):483–494
Akin M, Eyduran SP, Eyduran E, Reed BM (2020) Analysis of macro nutrient related growth responses using multivariate adaptive regression splines. Plant Cell Tissue Organ Cult 140(3):661–670
Kovalchuk IY, Mukhitdinova Z, Turdiyev T, Madiyeva G, Akin M, Eyduran E, Reed BM (2017) Modeling some mineral nutrient requirements for micropropagated wild apricot shoot cultures. Plant Cell Tissue Organ Cult 129(2):325–335
Kovalchuk IY, Mukhitdinova Z, Turdiyev T, Madiyeva G, Akin M, Eyduran E, Reed BM (2018) Nitrogen ions and nitrogen ion proportions impact the growth of apricot (Prunus armeniaca) shoot cultures. Plant Cell Tissue Organ Cult 133(2):263–273
Niedz RP, Evens TJ (2008) The effects of nitrogen and potassium nutrition on the growth of nonembryogenic and embryogenic tissue of sweet orange (Citrus sinensis (L.) Osbeck). BMC Plant Biol 8(1):1–1
Acknowledgements
The authors would like to thank all those who work in the Department of Agronomy and Plant Breeding, Campus of Agriculture and Natural Resources for their help. Financial support for this work has been provided by Razi University Grants No. 56747.
Funding
No external funding was obtained for this project.
Author information
Authors and Affiliations
Contributions
All authors have contributed equally to this research. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
None.
Research involving human and animal participants
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amani, M.R., Zebarjadi, A., Kahrizi, D. et al. Somatic embryogenesis and β-glucuronidase transformation in chickpea (Cicer arietinum cv. Bivanich). Mol Biol Rep 49, 11219–11227 (2022). https://doi.org/10.1007/s11033-022-07450-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07450-w