Abstract
Background
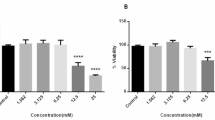
Normal cells produce energy (ATP) through mitochondrial oxidative phosphorylation in the presence of oxygen. However, many of the cancer cells produce energy with accelerated glycolysis and perform lactic acid production even under normoxic conditions called “The Warburg Effect”. In this study, human lung carcinoma cells (A549) were incubated in either a normoxic or hypoxic environment containing 5 mM glucose (Glc 5), 25 mM glucose (Glc 25), or 10 mM galactose (OXPHOS/aglycemic), and then the bioenergetic pathway was anaylsed.
Methods and results
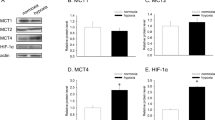
HIF-1α stabilization of A549 cells with different metabolic conditions in normoxia and hypoxia (1% O2) was determined using the western blot method. After that, l-lactic acid analysis, p-PDH/PDH expression ratio, ATP analysis, and citrate synthase activity experiments were also performed. It was determined that HIF-1α stabilization reached the maximum level at the 4 h. It has been found that glycolytic cells produce approximately five times more lactate than OXPHOS cells under both normoxia and hypoxia conditions and also have a higher p-PDH/PDH ratio. It has been determined that citrate synthase activity in hypoxia of all metabolic conditions is lower than normoxia. It has been determined that Glc 5 and Glc 25 cells have more ATP production under normoxia than Glc 5 and Glc 25 cells in hypoxia. OXPHOS cells have showed more ATP production in hypoxia.
Conclusion
It has been determined that oxidative phosphorylation became functional in a hypoxic aglycemic environment despite the metabolic programming regulated by HIF-1α. This data is important in determining targets for therapeutic intervention.






Similar content being viewed by others
Data availability
The data that support this study are available from the corresponding author upon reasonable request.
References
Semenza GL (2008) Tumor metabolism: cancer cells give and take lactate. J Clin Investig 118(12):3835–3837
Warburg O, Posener K, Negelein E (1924) Über den stoffwechsel der carcinomzelle. Naturwissenschaften 12(50):1131–1137
Warburg O (1925) The metabolism of carcinoma cells. J Cancer Res 9(1):148–163
Harris AL (2002) Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer 2(1):38–47
Semenza GL (2000) Surviving ischemia: adaptive responses mediated by hypoxia-inducible factor 1. J Clin Investig 106(7):809–812
Bahadori B, Uitz E, Mayer A, Harauer J, Dam K, Truschnig-Wilders M et al (2010) Polymorphisms of the hypoxia-inducible factor 1 gene and peripheral artery disease. Vascular Med 15(5):371–374
Sodhi A, Montaner S, Patel V, Zohar M, Bais C, Mesri EA et al (2000) The Kaposi’s sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1α. Cancer Res 60(17):4873–4880
Stiehl DP, Jelkmann W, Wenger RH, Hellwig-Bürgel T (2002) Normoxic induction of the hypoxia-inducible factor 1α by insulin and interleukin-1β involves the phosphatidylinositol 3-kinase pathway. FEBS Lett 512(1–3):157–162
Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ et al (2000) The expression and distribution of the hypoxia-inducible factors HIF-1α and HIF-2α in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol 157(2):411–421
Tian H, McKnight SL, Russell DW (1997) Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 11(1):72–82
Liu L, Ning X, Sun L, Zhang H, Shi Y, Guo C et al (2008) Hypoxia-inducible factor‐1α contributes to hypoxia‐induced chemoresistance in gastric cancer. Cancer Sci 99(1):121–128
Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D et al (1999) Overexpression of hypoxia-inducible factor 1α in common human cancers and their metastases. Cancer Res 59(22):5830–5835
Liao SH, Zhao XY, Han YH, Zhang J, Wang LS, Xia L et al (2009) Proteomics-based identification of two novel direct targets of hypoxia‐inducible factor‐1 and their potential roles in migration/invasion of cancer cells. Proteomics 9(15):3901–3912
Wenger RH, Stiehl DP, Camenisch G (2005) Integration of oxygen signaling at the consensus HRE. Science’s STKE 306:re-12
Hitosugi T, Fan J, Chung T-W, Lythgoe K, Wang X, Xie J et al (2011) Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism. Mol Cell 44(6):864–877
Krall AS, Christofk HR (2013) A metabolic metamorphosis. Nature 496(7443):38–40
Kim JW, Tchernyshyov I, Semenza GL, Dang CV (2006) HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metabol 3(3):177–185
Altman BJ, Stine ZE, Dang CV (2016) From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer 16(10):619–634
DeBerardinis RJ, Chandel NS (2016) Fundamentals of cancer metabolism. Sci Adv 2(5):e1600200
Jin L, Alesi G, Kang S (2016) Glutaminolysis as a target for cancer therapy. Oncogene 35(28):3619–3625
Smolková K, Plecitá-Hlavatá L, Bellance N, Benard G, Rossignol R, Ježek P (2011) Waves of gene regulation suppress and then restore oxidative phosphorylation in cancer cells. Int J Biochem Cell Biol 43(7):950–968
Vander Heiden MG, DeBerardinis RJ (2017) Understanding the intersections between metabolism and cancer biology. Cell 168(4):657–669
Rossignol R, Gilkerson R, Aggeler R, Yamagata K, Remington SJ, Capaldi RA (2004) Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res 64(3):985–993
Coller HA (2014) Is cancer a metabolic disease? Am J Pathol 184(1):4–17
Plecitá-Hlavatá L, Ježek J, Ježek P (2015) Aglycemia keeps mitochondrial oxidative phosphorylation under hypoxic conditions in HepG2 cells. J Bioenerg Biomembr 47(6):467–476
Ježek J, Plecitá-Hlavatá L, Ježek P (2018) Aglycemic HepG2 cells switch from aminotransferase glutaminolytic pathway of pyruvate utilization to complete Krebs cycle at hypoxia. Front Endocrinol 9:637
Nguyen LK, Cavadas MA, Scholz CC, Fitzpatrick SF, Bruning U, Cummins EP et al (2013) A dynamic model of the hypoxia-inducible factor 1α (HIF-1α) network. J Cell Sci 126(6):1454–1463
Yu AY, Frid MG, Shimoda LA, Wiener CM, Stenmark K, Semenza GL (1998) Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor-1 in the lung. Am J Physiology-Lung Cell Mol Physiol 275(4):L818–L26
Sato M, Tanaka T, Maeno T, Sando Y, Suga T, Maeno Y et al (2002) Inducible expression of endothelial PAS domain protein-1 by hypoxia in human lung adenocarcinoma A549 cells: role of Src family kinases-dependent pathway. Am J Respir Cell Mol Biol 26(1):127–134
Wiesener M, Turley H, Allen W, Willam C, Eckardt K-U, Talks K et al (1998) Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1α. Blood 92(7):2260–2268
Wiesener MS, Jürgensen JS, Rosenberger C, Scholze C, Hörstrup JH, Warnecke C et al (2003) Widespread, hypoxia-inducible expression of HIF‐2α in distinct cell populations of different organs. FASEB J 17(2):271–273
Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DA, Bauer C et al (2001) HIF-1 is expressed in normoxic tissue and displays an organ‐specific regulation under systemic hypoxia. FASEB J 15(13):2445–2453
Uchida T, Rossignol F, Matthay MA, Mounier R, Couette S, Clottes E et al (2004) Prolonged hypoxia differentially regulates hypoxia-inducible factor (HIF)-1α and HIF-2α expression in lung epithelial cells: implication of natural antisense HIF-1α. J Biol Chem 279(15):14871–14878
Graven KK, Bellur D, Klahn BD, Lowrey SL, Amberger E (2003) HIF-2α regulates glyceraldehyde-3-phosphate dehydrogenase expression in endothelial cells. Biochim Biophys Acta 1626(1–3):10–8
Wenger RH, Kvietiko I, Rolfs A, Gassmann M, Marti HH (1997) Hypoxia-inducible factor-1α is regulated at the post-mRNA level. Kidney Int 51(2):560–563
Semenza GL, Jiang B-H, Leung SW, Passantino R, Concordet J-P, Maire P et al (1996) Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 271(51):32529–32537
Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3(10):721–732
Semenza GL (2007) Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J 405(1):1–9
Ježek P, Plecitá-Hlavatá L, Smolková K, Rossignol R (2010) Distinctions and similarities of cell bioenergetics and the role of mitochondria in hypoxia, cancer, and embryonic development. Int J Biochem Cell Biol 42(5):604–622
Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D et al (2007) Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiology-Cell Physiol 292(1):C125–C36
Wigfield S, Winter S, Giatromanolaki A, Taylor J, Koukourakis M, Harris A (2008) PDK-1 regulates lactate production in hypoxia and is associated with poor prognosis in head and neck squamous cancer. Br J Cancer 98(12):1975–1984
Tang M, Etokidem E, Lai K (2016) The Leloir pathway of galactose metabolism–a novel therapeutic target for hepatocellular carcinoma. Anticancer Res 36(12):6265–6271
Morava E (2014) Galactose supplementation in phosphoglucomutase-1 deficiency; review and outlook for a novel treatable CDG. Mol Genet Metab 112(4):275–279
Heerlein K, Schulze A, Hotz L, Bartsch P, Mairbaurl H (2005) Hypoxia decreases cellular ATP demand and inhibits mitochondrial respiration of a549 cells. Am J Respir Cell Mol Biol 32(1):44–51
Funding
This work was funded by a grant from the Anadolu University (Project Nos. 1809S298, 1905S059).
Author information
Authors and Affiliations
Contributions
YÖK—Data analyzing and draft manuscript preperation, ZS—Supervision of the research and critical revison of the paper, YÖK and ZS—Final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest. The authors are fully responsible for data collection and extraction, interpretation, and writing of this manuscript.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Öğünç Keçeci, Y., İncesu, Z. Mitochondrial oxidative phosphorylation became functional under aglycemic hypoxia conditions in A549 cells. Mol Biol Rep 49, 8219–8228 (2022). https://doi.org/10.1007/s11033-022-07400-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07400-6