Abstract
Background
Noise-induced hearing loss (NIHL) is one the major causes of acquired hearing loss in developed countries. Noise can change the pattern of gene expression, inducing sensorineural hearing impairment. There is no investigation on the effects of noise frequency on the expression of GJB2 and SLC26A4 genes involved in congenital hearing impairment in cochlear tissue. Here we investigated the impacts of white and purple noise on gene expression and pathologic changes of cochlear tissue.
Methods
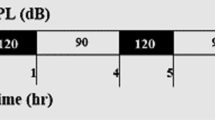
In this study, 32 adult male Westar rats were randomly divided into experimental groups: WN, animals exposed to white noise with a frequency range of 100-20000 Hz; PN, animals exposed to purple noise with a frequency range of 4–20 kHz, and control group, without noise. The experimental groups were exposed to a 118–120 dB sound pressure level for 8 h per 3 days and 6 days. 1 h and 1 week after termination of noise exposure, cochlear tissue was prepared for pathology and gene expression analysis.
Results
Both white and purple noises caused permanent damage to the cortical, estrosilica systems of hair cells and ganglion of the hearing nerve. GJB2 and SLC26A4 were downregulated in both groups exposed with white and purple noise by increasing the time of noise exposure. However, differences are notably more significant in purple noise, which is more intensified. Also, 1 weak post noise exposure, the downregulation is remarkably higher than 1 h.
Conclusions
Our findings suggest that downregulation of GJB2 and SLC26A4 genes are associated with pathological injury in response to noise exposure in cochlear tissue. It would be suggested the demand for assessment of RNA and protein expression of genes involved in noise-induced hearing loss and subsequently the practice of hearing protection programs.




Similar content being viewed by others
Availability of data and materials
Data will be available on special request to corresponding author.
Abbreviations
- GJB2:
-
Gap junction beta-2
- SLC26A4:
-
Solute carrier family 26 member 4
- NIHL:
-
Noise-induced hearing loss
- PN:
-
Purple noise
- WN:
-
White noise
- dB:
-
Decibel
- SIL:
-
Sound intensity level
- SPL:
-
Sound pressure level
- GADD45β:
-
Growth arrest and DNA damage inducible protein 45β
- CDK:
-
Cyclin-dependent kinase
- CIITA:
-
Class II transactivator
- MHCII:
-
Major histocompatibility complex II
- Cx26:
-
Connexin26
- CRH:
-
Corticotropin-Releasing Hormone
- Cfi:
-
Complement factor I
- C1s:
-
Complement component 1, s subcomponent
- IHC:
-
Inner hair cells
- OHC:
-
Outer hair cells
- PTS:
-
Permanent threshold shifts
- PDS:
-
Pendred syndrome
- PFA:
-
Paraformaldehyde
- H&E:
-
Hematoxylin-Eosin
- dNTP:
-
Deoxy Nucleoside Triphosphate
- RT:
-
Reverse transcriptase
- GAPDH:
-
Glyceraldehyde 3-Phosphate Dehydrogenase
- ΔΔCt:
-
Delta-delta cycle threshold
- HSP:
-
Heat shock proteins
- MAPK:
-
Mitogen-activated protein kinase
- CRH-R:
-
Corticotropin-releasing hormone receptor
- SOD2:
-
Superoxide dismutase 2
- CAT:
-
catalase
- CX26:
-
Connexin 26
- PTS:
-
Permanent hearing threshold
References
Sliwinska-Kowalska M, Davis A (2012) Noise-induced hearing loss. Noise Health 14(61):274–280. https://doi.org/10.4103/1463-1741.104893
Alagramam KN, Stepanyan R, Jamesdaniel S, Chen DH, Davis RR (2014) Noise exposure immediately activates cochlear mitogen-activated protein kinase signaling. Noise health 16(73):400–409. https://doi.org/10.4103/1463-1741.144418
Park SN, Back SA, Park KH, Seo JH, Noh HI, Akil O et al (2013) Comparison of functional and morphologic characteristics of mice models of noise-induced hearing loss. Auris Nasus Larynx 40(1):11–17. https://doi.org/10.1016/j.anl.2011.11.008
ACOEM Task Force on Occupational Hearing Loss, Kirchner DB, Evenson E, Dobie RA, Rabinowitz P, Crawford J, Kopke R et al (2012) Occupational noise-induced hearing loss: ACOEM task force on occupational hearing loss. J Occup Environ Med 54(1):106–108. https://doi.org/10.1097/JOM.0b013e318242677d
Lu SY, Huang YH, Lin KY (2020) Spectral content (color) of noise exposure affects work efficiency. Noise Health 22(104):19–27. https://doi.org/10.4103/nah.NAH_61_18
Ninomiya H, Ohgami N, Oshino R, Kato M, Ohgami K, Li X et al (2018) Increased expression level of Hsp70 in the inner ears of mice by exposure to low frequency noise. Hear Res 363:49–54. https://doi.org/10.1016/j.heares.2018.02.006
Gratton MA, Eleftheriadou A, Garcia J, Verduzco E, Martin GK, Lonsbury–Martin BL et al (2011) Noise-induced changes in gene expression in the cochleae of mice differing in their susceptibility to noise damage. Hear Res 277(1–2):211–226. https://doi.org/10.1016/j.heares.2010.12.014
Yang W, Vethanayagam RR, Dong Y, Cai Q, Hu BH (2015) Activation of the antigen presentation function of mononuclear phagocyte populations associated with the basilar membrane of the cochlea after acoustic overstimulation. Neuroscience 30(3):1–15. https://doi.org/10.1016/j.neuroscience.2015.05.081
Zhou X-X, Chen S, Xie L, Ji Y-Z, Wu X, Wang W-W et al (2016) Reduced Connexin26 in the mature cochlea increases susceptibility to noise-induced hearing loss in mice. Int J Mol Sci 17(3):301. https://doi.org/10.3390/ijms17030301
Patel M, Hu Z, Bard J, Jamison J, Cai Q, Hu BH (2013) Transcriptome characterization by RNA-Seq reveals the involvement of the complement components in noise-traumatized rat cochleae. Neuroscience 248:1–16. https://doi.org/10.1016/j.neuroscience.2013.05.038
Du F, Yin L, Shi M, Cheng H, Xu X, Liu Z et al (2010) Involvement of microglial cells in infrasonic noise-induced stress via upregulated expression of corticotrophin releasing hormone type 1 receptor. Neuroscience 167(3):909–919. https://doi.org/10.1016/j.neuroscience.2010.02.060
Chen G-D (2006) Prestin gene expression in the rat cochlea following intense noise exposure. Hear Res 222(1–2):54–61. https://doi.org/10.1016/j.heares.2006.08.011
Manikandan S, Srikumar R, Parthasarathy NJ, Devi RS (2005) Protective effect of acorus calamus L INN on free radical scavengers and lipid peroxidation in discrete regions of brain against noise stress exposed rat. Biol Pharm Bull 28(12):2327–2330. https://doi.org/10.1248/bpb.28.2327
Han Y, Wang X, Chen J, Sha SH (2015) Noise-induced cochlear F‐actin depolymerization is mediated via ROCK 2/p‐ERM signaling. J Neurochem 133(5):617–628. https://doi.org/10.1111/jnc.13061
Chen GD, Fechter LD (2003) The relationship between noise-induced hearing loss and hair cell loss in rats. Hear Res 177(1–2):81–90. https://doi.org/10.1016/s03785955(02)00802-x
Hamernik RP, Qiu W (2000) Correlations among evoked potential thresholds, distortion product optoacoustic emissions and hair cell loss following various noise exposures in the chinchilla. Hear Res 150(1–2):245–257. https://doi.org/10.1016/s0378-5955(00)00204-5
Chen YS, Liu TC, Cheng CH, Yeh TH, Lee SY, Hsu CJ (2003) Changes of hair cell stereocilia and threshold shift after acoustic trauma in guinea pigs: comparison between inner and outer hair cells. ORL J Otorhinolaryngol Relat Spec 65(5):266–274. https://doi.org/10.1159/000075224
Hu BH, Henderson D, Nicotera TM (2006) Extremely rapid induction of outer hair cell apoptosis in the chinchilla cochlea following exposure to impulse noise. Hear Res 211(1–2):16–25. https://doi.org/10.1016/j.heares.2005.08.006
Bohne BA, Harding GW, Lee SC (2007) Death pathways in noise damaged outer hair cells. Hear Res 223(1–2):61–70. https://doi.org/10.1016/j.heares.2006.10.004
Harding GW, Bohne BA (2009) Relation of focal hair-cell lesions to noise exposure parameters from a4- or a 0.5- kHz octave band of noise. Hear Res 254(1–2):54–63. https://doi.org/10.1016/j.heares.2009.04.011
Yang S, Cai Q, Vethanayagam RR, Wang J, Yang W, Hu BH (2016) Immune defense is the primary function associated with the differentially expressed genes in the cochlea following acoustic trauma. Hear Res 33(3):283–294. https://doi.org/10.1016/j.heares.2015.10.010
Mei L, Chen J, Zong L, Zhu Y, Liang C, Jones RO, Zhao HB (2017) A deafness mechanism of digenic Cx26 (GJB2) and Cx30 (GJB6) mutations: Reduction of endocochlear potential by impairment of heterogeneous gap junctional function in the cochlear lateral wall. Neurobiol Dis 108:195–203. https://doi.org/10.1016/j.nbd.2017.08.002
Martins FTA, Ramos PZ, Svidnicki MCCM, Castilho AM, Sartorato EL (2013) Optimization of simultaneous screening of the main mutations involved in non-syndromic deafness using the TaqMan® OpenArray™ Genotyping Platform. BMCMed Genet 14:112. https://doi.org/10.1186/1471-2350/14/112
Nonose RW, Lezirovitz K, de Mello Auricchio MTB, Batissoco AC, Yamamoto GL, Mingroni-Netto RC (2018) Mutation analysis of SLC26A4 (Pendrin) gene in a Brazilian sample of hearing-impaired subjects. BMC Med Genet 19(1):73. https://doi.org/10.1186/s12881-018-0585-x
Gong T-W, Fairfield DA, Fullarton L, Dolan DF, Altschuler RA, Kohrman DC, Lomax MI (2012) Induction of heat shock proteins by hyperthermia and noise overstimulation in Hsf1–/– mice. J Assoc Res Otolaryngol 13(1):29–37. https://doi.org/10.1007/s10162-011-0289-9
Kommareddi P, Nair T, Kakaraparthi BN, Galano MM, Miller D, Laczkovich I et al (2015) Hair cell loss, spiral ganglion degeneration, and progressive sensorineural hearing loss in mice with targeted deletion of Slc44a2/Ctl2. J Assoc Res Otolaryngol 16(6):695–712. https://doi.org/10.1007/s10162-015-0547-3
Mohebali M, Kazemirad E, Hajjaran H, Kazemirad E, Oshaghi MA, Raoofian R, Teimouri A (2019) Gene expression analysis of antimony resistance in Leishmania tropica using quantitative real-time PCR focused on genes involved in trypanothione metabolism and drug transport. Arch Dermatol Res 311(1):9–17. https://doi.org/10.1007/s00403-018-1872-2
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–∆∆ct Method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001
Hsu WC, Wang JD, Hsu CJ, Lee SY, Yeh TH (2004) Expression of connexin 26 in the lateral wall of the rat cochlea after acoustic trauma. Acta Otolaryngol 124(4):459–463. https://doi.org/10.1080/00016480310000584
Durán Alonso MB, Vendrell V, López-Hernández I, Alonso MT, Martin DM, Giráldez F, Carramolino L, Giovinazzo G, Vázquez E, Torres M, Schimmang T (2021) Meis2 Is Required for Inner Ear Formation and Proper Morphogenesis of the Cochlea. Front Cell Dev Biol 9:679325. https://doi.org/10.3389/fcell.2021.679325
Chance MR, Chang J, Liu S, Gokulrangan G, Chen DH, Lindsay A, Geng R, Zheng QY, Alagramam K (2010) Proteomics, bioinformatics and targeted gene expression analysis reveals up-regulation of cochlin and identifies other potential biomarkers in the mouse model for deafness in Usher syndrome type 1F. Hum Mol Genet 15;19(8):1515-27. https://doi.org/10.1093/hmg/ddq025
Melgar-Rojas P, Alvarado JC, Fuentes-Santamaría V, Gabaldón-Ull MC, Juiz JM (2015) Validation of Reference Genes for RT-qPCR Analysis in Noise-Induced Hearing Loss: A Study in Wistar Rat. PLoS ONE 10(9):e0138027. https://doi.org/10.1371/journal.pone.0138027
Sun W, Zhang L, Lu J, Yang G, Laundrie E, Salvi R (2008) Noise exposure–induced enhancement of auditory cortex response and changes in gene expression. Neuroscience 156(2):374–380. https://doi.org/10.1016/j.neuroscience.2008.07.040
Eraslan E, Akyazi I, Ergül-Ekiz E, Matur E (2015) Noise stress changes mRNA expressions of corticotropin-releasing hormone, its receptors in amygdala, and anxiety-related behaviors. Noise health 17(76):141. https://doi.org/10.4103/1463-1741.155838
Wang SL, Yu LG, Liu RP, Zhu WZ, Gao WM, Xue LP et al (2014) Gene-gene interaction of GJB2, SOD2, and CAT on occupational noise-induced hearing loss in Chinese Han population. Biomed Environ Sci 27(12):965–968. https://doi.org/10.3967/bes2014.131
Sun D, Li X (2000) The effect of noise exposure on the expression of NOSmRNA in the cochlea. Lin chuang er bi yan hou ke za zhi. J Clin Otorhinolaryngol 14(8):373–374 PMID:12563903
Daiber A, Kröller-Schön S, Oelze M, Hahad O, Li H, Schulz R, Steven S, Münzel T (2020) Oxidative stress and inflammation contribute to traffic noise-induced vascular and cerebral dysfunction via uncoupling of nitric oxide synthases. Redox Biol 34:101506. https://doi.org/10.1016/j.redox.2020.101506
Monsefi M, Talaei T (2005) Changes of heart glycoconjugates by noise stress in mouse as an experimental model. J Appl Anim Res 27(2):121–124. https://doi.org/10.1080/09712119.2005.9706554
Chorath K, Willis M, Morton-Gonzaba N, Moreira A (2020) Mesenchymal stem cells for sensorineural hearing loss: a systematic review of preclinical studies. Mol Biol Rep 47(4):4723–4736. https://doi.org/10.1007/s11033-020-05460-0
Acknowledgements
The authors would like to express their gratitude to the Assistance of Research and Technology at Tehran University of Medical Sciences.
Funding
This research was supported by Tehran University of Medical Sciences & Health Services, Grant Numbers: 98-3-99-45886.
Author information
Authors and Affiliations
Contributions
AAG, MRME and EK collected and analyzed data. AAG and EK wrote the manuscript. MK, MMH and SM reviewed and revised the manuscript. EK and MRME supervised the entire processes. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest/Competing interests
The authors declare that they have no competing of interest.
Ethical approval and consent to participate
The study was approved by the Ethics Research Committee of the School of Public Health, Tehran University of Medical Sciences (Code: IR.TUMS.SPH.REC.1398.220).
Consent for publication
All authors read and approved the final manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garmaroudi, A.A., Khadem, M., Hotkani, M.M. et al. Downregulation of GJB2 and SLC26A4 genes induced by noise exposure is associated with cochlear damage. Mol Biol Rep 49, 7219–7229 (2022). https://doi.org/10.1007/s11033-022-07291-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07291-7