Abstract
Background
Transforming growth factor beta (TGF-β) superfamily has key role in cell proliferation which leads to tumor promoting activities at metastatic stage of cancer. Inhibition of transforming growth factor beta receptor (TGFβR) signaling pathway can provide better therapeutic strategy to control cancer. Natural products are best known for their safety, less toxic nature, antioxidant characteristics making them a promising candidate to inhibit TGFβR signaling pathway.
Methods and Results
Crude methanolic extracts (CMEs) of 16 selected plants were prepared by using maceration method and subjected to phytochemical assays for identification of major phytometabolites particularly cancer chemopreventive antioxidant constituents. Total flavonoid content of all plants CME was > 0.6 mg/ml exhibiting the Cichorium intybus contains comparatively highest amount of total flavonoid content (0.53 mg/ml). Scanvenging activity of all plants was determined having IC50 ranges between 2 and 88 (µg/ml) while Moringa oleifera revealed the maximum scavenging activity (IC50 2.03 µg/ml).
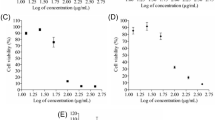
Comparative cytotoxicity of plant extracts was evaluated in HUH and MCF-7 cell lines using 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide (MTT) colorimetric assay. The nine active plant extracts i.e. Fagonia cretica, Argemone Mexicana, Rubus fruticosus, M. oleifera, Punica granatum, Cichorium intybus, Xanthium strumarium, Carissa opaca, Cyperus rotundus were identified based on their high antiproliferative activity > 50% against cancer cell lines and subjected to relative expression studies. Modulation of TGFβ signaling molecules (i.e.TGFβR1, 2 & 3, SMAD3, SMAD5) and ubiquitous proteins i.e. SMURF1 and SMURF2 genetic expression by potent extracts was determined by RT-PCR using GAPDH (housekeeping gene) as gene of reference.
Conclusions
This present study revealed that CME of Fagonia cretica and Argemone mexicana significantly inhibit TGF beta mediated signaling cascade by downregulating the gene expression fold change > 1 of TGFβR 1, 2 & 3 and receptor associated complex protein SMAD3 as compared to control.




Similar content being viewed by others
References
David CJ, Massague J (2018) Contextual determinants of TGFbeta action in development, immunity and cancer. Nat Rev Mol Cell Biol 19:419–435
Li Y, Zhang B, Xiang L, Xia S, Kucuk O, Deng X et al (2020) TGF-beta causes docetaxel resistance in prostate cancer via the induction of Bcl-2 by acetylated KLF5 and protein stabilization. Theranostics 10:7656–7670
Miyazono K, Maeda S, Imamura T (2005) BMP receptor signaling transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev 16(3):251–263
Biswas S, Guix M, Rinehart C, Dugar TC, Chytil A, Moses HL, Freeman ML, Arteaga CL (2007) Inhibition of TGF-β with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest 117(5):1305–1313
Massagué J (2012) TGFβ signaling in context. Nature reviews Mol Cell Biol 13(10):616–630
Singh P, Singh RS, Rani A, Bast F (2016) Homology modeling of chemokine CCR7, molecular docking, and in vitro studies evidenced plausible immunotherapeutic anticancer natural compounds. Med Chem Res 25(10):2410–2424
Jia L, Jin H, Zhou J, Chen L, Lu Y, Ming Y, Yu Y (2013) A potential anti-tumor herbal medicine, Corilagin, inhibits ovarian cancer cell growth through blocking the TGF-β signaling pathways. BMC Complement Altern Med 13(1):1–1
Batool S, Ullah S, Tabassum S, Bilal A, Faisal A, Saleem RS, Ahmad MS (2019) Effect of extraction methodologies on antioxidant potential and synergistic anticancer behavior of leaf extract of Polygonum amplexicaule against HCT-116 cells. Iran J Sci Technol Trans A Sci 43(3):709–714
Tabassam S, Anwar MA, Gulfraz M, Arshad M, Sabitaliyevich UY, Nurmurzayevich SB, Ahmad MS (2019) Bioactivity evaluation and HPLC UV-VIS based quantification of antioxidant secondary metabolites from extract and fractions of Bistorta amplexicaulis rhizome. Mol Cell Biol 65(1):19–26
Sakanaka S, Tachibana Y, Okada Y (2005) Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem 89(4):569–575
Patel A, Patel A, Patel A, Patel NM (2010) Estimation of flavonoid, polyphenolic content and in vitro antioxidant capacity of leaves of Tephrosia purpurea Linn (Leguminosae). Int J Pharma Sci and Res 1(1):66–77
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C T method. Nat Protoc 3(6):1101
Mo N, Li ZQ, Li J, Cao YD (2012) Curcumin inhibits TGF-β1-induced MMP-9 and invasion through ERK and Smad signaling in breast cancer MDA-MB-231 cells. Asian Pac J Cancer Prev 13(11):5709–5714
Wang H, Zhang H, Tang L, Chen H, Wu C, Zhao M, Yang Y, Chen X, Liu G (2013) Resveratrol inhibits TGF-β1-induced epithelial-to-mesenchymal transition and suppresses lung cancer invasion and metastasis. Toxicology 303:139–146
Wang SD, Chen BC, Kao ST, Liu CJ, Yeh CC (2014) Genistein inhibits tumor invasion by suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. BMC Complement Altern Med 14(1):26
Izzi L, Attisano L (2004) Regulation of the TGF β signalling pathway by ubiquitin-mediated degradation. Oncogene 23(11):2071–2078
Pourmorad F, Hosseinimehr SJ, Shahabimajd N (2006) Antioxidant activity phenol and flavonoid contents of some selected Iranian medicinal plants. Afr J Biotechnol 5(11):1142–1145
Koleva II, Van Beek TA, Linssen JP, Groot AD, Evstatieva LN (2002) Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal 13(1):8–17
Batra P, Sharma AK (2013) Anti-cancer potential of flavonoids recent trends and future perspectives. 3 Biotech 3(6):439–59
Vaishnav K, George LB, Highland HN (2015) Antitumour activity of Xanthium strumarium L. on human cervical cancer HeLa cells. Int J Cancer 23:1–3
Gutiérrez RM, Mitchell S, Solis RV (2008) Psidium guajava: a review of its traditional uses, phytochemistry and pharmacology. J Ethno Pharmacol 117(1):1–27
Mawa S, Husain K, Jantan I (2013) Ficus carica L.(Moraceae): phytochemistry, traditional uses and biological activities. Evid Based Complement Altern Med 2013
Meghwal M, Goswami TK (2013) Piper nigrum and piperine: an update. Phytother Res 27(8):1121–1130
Satpute R, Bhattacharya R, Kashyap RS, Purohit HJ, Deopujari JY, Taori GM, Daginawala HF (2012) Antioxidant potential of Fagonia arabica against the chemical ischemia - induced in PC12 cells. Iran J Pharm Res 11(1):303
Kumar VS, Navaratnam V (2013) Neem (Azadirachta indica): prehistory to contemporary medicinal uses to humankind. Asian Pac J Trop Biomed 3(7):505–514
Singh A, Jain D, Upadhyay MK, Khandelwal N, Verma HN (2010) Green synthesis of silver nanoparticles using Argemone mexicana leaf extract and evaluation of their antimicrobial activities. Dig J Nanomater Biostruct 5(2):483–489
Zia-Ul-Haq M, Riaz M, Feo VD, Jaafar HZ, Moga M (2014) Rubus fruticosus L.: constituents, biological activities and health related uses. Molecules 19(8):10998–11029
Apostolou A, Stagos D, Galitsiou E, Spyrou A, Haroutounian S, Portesis N, Trizoglou I, Hayes AW, Tsatsakis AM, Kouretas D (2013) Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of Vitis vinifera stem extracts. Food Chem Toxicol 61(1):60–68
Farooq F, Rai M, Tiwari A, Khan AA, Farooq S (2012) Medicinal properties of Moringa oleifera: an overview of promising healer. J Med Plant Res 6(27):4368–4374
Zhao G, Yin Z, Dong J (2009) Antiviral efficacy against hepatitis B virus replication of oleuropein isolated from Jasminum officinale L. var. grandiflorum. J Ethnopharmacol 125(2):265–268
Arun N, Singh DP (2012) Punica granatum: a review on pharmacological and therapeutic properties. Int J Pharm Res 1;3(5):1240
Street RA, Sidana J, Prinsloo G (2013) Cichorium intybus: Traditional uses, phytochemistry, pharmacology, and toxicology. Evid Based Complementary Altern Med 2013
Ramírez-Erosa I, Huang Y, Hickie RA, Sutherland RG, Barl B (2007) Xanthatin and xanthinosin from the burs of Xanthium strumarium L. as potential anticancer agents. Can J Physiol Pharmacol 85(11):1160–1172
Sahreen S, Khan MR, Khan RA, Shah NA (2013) Estimation of flavoniods, antimicrobial, antitumor and anticancer activity of Carissa opaca fruits. BMC Complement Altern Med 13(1):1–7
Acknowledgements
Dr. Rahmatullah Qureshi for identification of plants. Assitant Professor Dr. Tayyaba Zainab and Professor Dr. Mazhar Qayyum for supervising study design review and approval. The financial support from Higher education commission (HEC), Pakistan through NRPU Project No. 10448.
Author information
Authors and Affiliations
Contributions
AJ: Study conception, review of literature experimental work, data analysis, results interpretation, data compilation and article write-up. QM: Study validation, experimental work guidance, infrastructure for in-vitro experimentation and gene expression, data analysis, results interpretation and article compilation and article submission. TZ: Study design review and approval. AAF: Gene expression analysis and comments. MQ: Study review and approval, Laboratory infrastructure provision for plant extracts preparation. AS: Phytochemical analysis experiments. AM: Antioxidant assays experiments. MSA: Study Design, approval, ethical considerations and funds management. Data interpretation, article compilation and author contribution acknowledgment.
Corresponding authors
Ethics declarations
Conflict of interest
The Authors of this article announce that they have no known contending monetary interests or individual connections that might have seemed to impact the work reported in this paper.
Consent to participate
All authors agreed upon the consent to fully participate in this publication.
Consent to Publication
All authors approved the consent for publication of this article in “Molecular Biology Reports”.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.






Rights and permissions
About this article
Cite this article
Jabeen, A., Mansoor, Q., Zainab, T. et al. Natural plant extracts mediated expression regulation of TGF-β receptors and SMAD genes in human cancer cell lines. Mol Biol Rep 49, 4171–4178 (2022). https://doi.org/10.1007/s11033-022-07250-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07250-2