Abstract
Background
Streptozotocin is a classic drug used to induce diabetes in animal models.
Objective
The aim of this study is to investigate the liver transcriptome of Kunming mice with diabetes induced by either streptozotocin (STZ) or Non-STZ.
Methods
Forty male mice were randomly assigned into four groups: Control (Ctr, standard diet), mHH (high fat and high carbohydrate diet), mHS (high fat and high carbohydrate diet for 4 weeks followed by 60 mg/kg STZ for 3 consecutive days) and mSH (60 mg/kg STZ for 3 consecutive days followed by a high fat and high carbohydrate diet for 12 weeks). All mice injected with STZ were identified as diabetic despite the sequential feeding of high fat and high carbohydrate diets.
Results
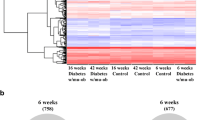
Only 7 of 13 mice in the mHH group met the diagnostic criteria for diabetes. The asting blood glucose (FBG) of the mHH, mHS, mSH and Ctrl groups was 13.27 ± 1.14, 15.01 ± 2.59, 15.95 ± 4.38 and 6.28 ± 0.33 mmol/L at the 12th week, respectively. Compared with the mHH group, transcription was elevated in 85 genes in the livers of mHS mice, while 21 genes were downregulated and 97 genes were upregulated in the mSH group while 35 genes were decreased. A total of 43 co-expressed genes were identified in the mHS vs mHH and mSH vs mHH groups. GO (Gene Ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes) analyses showed that two corporate GO terms and two KEGG pathways were significantly annotated in the STZ-treated groups. Both the GO term and pathway were related to the metabolism mediated by p53.
Conclusion
A high fat and high carbohydrate diet combined with a low dose of STZ can effectively induce diabetes in Kunming mice despite the abnormal expressions of genes in the liver. The differentially expressed genes were related to metabolism mediated by p53.



Similar content being viewed by others
Data availability
The underlying data supporting the results are available from the corresponding authors upon reasonable request.
References
Kahn SE, Hull RL, Utzschneider K (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444(7121):840–846
Wolf E, Braun-Reichhart C, Streckel E, Renner S (2014) Genetically engineered pig models for diabetes research. Transgenic Res 23(1):27–38
Kavanagh K, Flynn DM, Nelson C, Zhang L, Wagner JD (2011) Characterization and validation of a streptozotocin-induced diabetes model in the vervet monkey. J Pharmacol Toxicol Methods 63(3):296–303
Xi S, Yin W, Wang Z et al (2004) A minipig model of high-fat/high-sucrose diet-induced diabetes and atherosclerosis. Int J Exp Pathol 85(4):223–231
Liu Y, Wang Z, Yin W et al (2007) Severe insulin resistance and moderate glomerulosclerosis in a minipig model induced by high-fat/ high-sucrose/ high-cholesterol diet. Exp Anim 56(1):11–20
Emonnot L, Cohen R, Lo M (2007) Neonatal streptozotocin-induced glucose intolerance: different consequences in Lyon normotensive and hypertensive rats. J Hypertens 25(2):429–438
Ma H, You GP, Zhang XP et al (2014) A novel role of globular adiponectin in treatment with HFD/STZ induced T2DM combined with NAFLD rats. Sci World J 2014:230835
Gerrity RG, Natarajan R, Nadler JL, Kimsey T (2001) Diabetes-induced accelerated atherosclerosis in swine. Diabetes 50(7):1654–1665
Szkudelski T (2001) The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 50(6):537–546
Volk BW, Wellmann KF, Brancato P (1974) Fine structure of rat islet cell tumors induced by streptozotocin and nicotinamide. Diabetologia 10(1):37–44
Podell BK, Ackart DF, Richardson MA et al (2017) A model of type 2 diabetes in the guinea pig using sequential diet-induced glucose intolerance and streptozotocin treatment. Dis Model Mech 10(2):151–162
Kim JH, Lee DE, Choi SH et al (2014) Diabetic characteristics and alveolar bone loss in streptozotocin- and streptozotocin-nicotinamide-treated rats with periodontitis. J Periodontal Res 49(6):792–800
Prathaban S, Gnanaprakasam V (2000) Streptozotocin induced diabetes mellitus in dogs. Indian Vet J 77(3):219–222
Ganda OP, Rossini AA, Like AA (1976) Studies on streptozotocin diabetes. Diabetes 25(7):595–603
Katsumata K, Katsumata KJ, Katsumata Y (1992) Protective effect of diltiazem hydrochloride on the occurrence of alloxan- or streptozotocin-induced diabetes in rats. Horm Metab Res 24(11):508–510
Nie YW, Zhang P, Zhang J et al (2016) Isolation and characterization of white and brown adipocytes in Kunming mice. Genet Mol Res 15(1):15017355
Yu S, Yan X, Liu H et al (2014) Improved establishment of embryonic stem (ES) cell lines from the Chinese Kunming mice by hybridization with 129 mice. Int J Mol Sci 15(3):3389–3402
Xie H, Huang L, Li Y, Zhang H, Liu H (2016) Endoplasmic reticulum stress and renal lesion in mice with combination of high-fat diet and streptozotocin-induced diabetes. Acta Cir Bras 31(3):150–155
Wu F, Jin Z, Jin J (2013) Hypoglycemic effects of glabridin, a polyphenolic flavonoid from licorice, in an animal model of diabetes mellitus. Mol Med Rep 7(4):1278–1282
Wu Y, Zhang L, Liang J et al (2018) Comparative analysis on liver transcriptome profiles of different methods to establish type 2 diabetes mellitus models in Guangxi Bama mini-pig. Gene 673:194–200
Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P (2005) Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res 52(4):313–320
Kawashima Y, Chen J, Sun H et al (2009) Apolipoprotein E deficiency abrogates insulin resistance in a mouse model of type 2 diabetes mellitus. Diabetologia 52(7):1434–1441
Nagata M, Suzuki W, Iizuka S et al (2006) Type 2 diabetes mellitus in obese mouse model induced by monosodium glutamate. Exp Anim 55(2):109–115
Trapnell C, Pachter L, Salzberg SL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25(9):1105–1111
Trapnell C, Williams BA, Pertea G et al (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28(5):511–515
Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11(10):R106
Young MD, Wakefield MJ, Smyth GK, Oshlack A (2010) Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol 11(2):R14
Mao X, Cai T, Olyarchuk JG, Wei L (2005) Automated genome annotation and pathway identification using the KEGG orthology (KO) as a controlled vocabulary. Bioinformatics 21(19):3787–3793
Houssay BA, Martinez C (1947) Experimental diabetes and diet. Science 105(2734):548–549
Graham ML, Janecek JL, Kittredge JA, Hering BJ, Schuurman HJ (2011) The streptozotocin-induced diabetic nude mouse model: differences between animals from different sources. Comp Med 61(4):356–360
Skovso S (2014) Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Investig 5(4):349–358
Rakieten N, Rakieten ML, Nadkarni MR (1963) Studies on the diabetogenic action of streptozotocin (NSC-37917). Cancer Chemother Rep 29:91–8
Brosky G, Logothetopoulos J (1969) Streptozotocin diabetes in the mouse and guinea pig. Diabetes 18(9):606–611
Junod A, Lambert AE, Orci L et al (1967) Studies of the diabetogenic action of streptozotocin. Proc Soc Exp Biol Med 126(1):201–205
Rerup CC (1970) Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev 22(4):485–518
Reed MJ, Meszaros K, Entes LJ et al (2000) A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism 49(11):1390–1394
Gilbert ER, Fu Z, Liu D (2011) Development of a nongenetic mouse model of type 2 diabetes. Exp Diabetes Res 2011:416254
Goto Y, Nagasawa H, Iguchi T (1995) Streptozotocin-induced diabetes accelerates mammary tumorigenesis in shn and sln mice. Oncol Rep 2(1):37–40
Gruys ME, Back TC, Subleski J et al (2001) Induction of transplantable mouse renal cell cancers by streptozotocin: in vivo growth, metastases, and angiogenic phenotype. Cancer Res 61(16):6255–6263
Fienhold MA, Kazakoff K, Pour PM (1997) The effect of streptozotocin and a high-fat diet on BOP-induced tumors in the pancreas and in the submandibular gland of hamsters bearing transplants of homologous islets. Cancer Lett 117(2):155–160
Dombrowski F, Bannasch P, Pfeifer U (1997) Hepatocellular neoplasms induced by low-number pancreatic islet transplants in streptozotocin diabetic rats. Am J Pathol 150(3):1071–1087
Levine AJ (1997) p53, the cellular gatekeeper for growth and division. Cell 88(3):323–331
Kim MK, Jeon BN, Koh DI et al (2013) Regulation of the cyclin-dependent kinase inhibitor 1A gene (CDKN1A) by the repressor BOZF1 through inhibition of p53 acetylation and transcription factor Sp1 binding. J Biol Chem 288(10):7053–7064
El-Deiry WS, Tokino T, Velculescu VE et al (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75(4):817–825
El-Deiry WS, Harper JW, O’Connor PM et al (1994) WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res 54(5):1169–1174
Chua HW, Ng D, Choo S et al (2010) Effect of MDM2 SNP309 and p53 codon 72 polymorphisms on lung cancer risk and survival among non-smoking Chinese women in Singapore. BMC Cancer 10(1):88–88
Momand J, Zambetti GP, Olson DC, George D, Levine AJ (1992) The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 69(7):1237–1245
Funding
This work was supported by the Science and technology funds of the chairman of the Autonomous Region (No. 16449-10), the Science and Technology Major Special Project of Guangxi (No. Guike-AA17292002), the Guangdong Basic and Applied Basic Research Fund (2019A1515110280), the Foshan Science and Technology Innovation Project (1920001001203) and the Guangdong Science and Technology Innovation Strategy Fund (The Special Fund for “Climbing Plan”; pdjh2020a0616).
Author information
Authors and Affiliations
Contributions
GQL and YJW conceived and designed the experiments; YJW, XXZ and JL performed the experiments and analyzed the data; ASO, ZSL, JCW and DYG participated in experiment assistant. GQL, YJW, XXZ and SGW drafted the manuscript. All authors participated in discussions of the results and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Author Yanjun Wu declares that he has no conflict of interest. Author Xiangxing Zhu declares that he has no conflict of interest. Author Arome Solomon Odiba declares that he has no conflict of interest. Author Zisheng Lin declares that he has no conflict of interest. Author Jiancong Wen declares that he has no conflict of interest. Author Daoyuan Gong declares that he has no conflict of interest. Author Jing Liang declares that he has no conflict of interest. Author Shuguang Wu declares that she has no conflict of interest. Author Ganqiu Lan declares that he has no conflict of interest.
Ethical approval
All of the animal procedures used in this study were carried out in accordance with the Guide for Care and Use of Laboratory Animals (8th edition, released by the National Research Council, USA), and were approved by the Animal Care & Welfare Committee of Guizhou University of Traditional Chinese Medicine and Foshan University (Approval No. 2019020). All of the surgical procedures were performed under anesthesia by a veterinarian, and all efforts were made to minimize animal suffering.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, Y., Zhu, X., Odiba, A.S. et al. Comparatively analyzing the liver-specific transcriptomic profiles in Kunming mice afflicted with streptozotocin- and natural food-induced type 2 diabetes mellitus. Mol Biol Rep 49, 1369–1377 (2022). https://doi.org/10.1007/s11033-021-06970-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06970-1