Abstract
Background
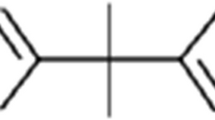
Bisphenol S (BPS) is a chemical compound that is utilized in the plastic industry as an alternative to bisphenol A (BPA). The toxic effects of BPS in fish is less known and limited. Therefore, in the present study, the influence of BPS on rainbow trout (Oncorhyncus mykiss) hepatocytes in vitro was investigated.
Methods and results
For this purpose the fish hepatocytes were isolated, and then the cultured cells were treated with increasing concentrations of BPS (0, 15.63, 31.25, 62.50, 125, 250, and 500 µM) for 24 h. The cytotoxic impact of BPS was determined in the culture media using lactate dehydrogenase assay and then, the antioxidant defence indicators were assayed. The results showed that concentration-dependent increases were observed in the percentage of cytotoxicity. The superoxide dismutase activity was reduced, while the catalase and glutathione peroxidase activity increased with all of the BPS concentrations. The glutathione S-transferase (GST) activity significantly increased after a BPS concentration of 31.25 µM or higher, while GST Theta 1-1 activity was decreased by the same concentrations of BPS. The reduced glutathione content significantly decreased with a BPS concentration of 31.25 µM or higher, and the malondialdehyde content increased after BPS concentrations of 125, 250, and 500 µM.
Conclusions
The findings determined herein suggested that BPS causes cytotoxicity in fish hepatocytes and can lead to oxidative stress, resulting hepatotoxic in fish. Thus, the utilization of BPS instead of BPA as safe alternative in industry should be re-evaluated in the future for environmental health.



Similar content being viewed by others
Data availability
All of the data and the material that were used and analyzed in the course of the study herein can be obtained from the author for correspondence upon reasonable request.
References
Seachrist DD, Bonk KW, Ho SM, Prins GS, Soto AM, Keri RA (2016) A review of the carcinogenic potential of bisphenol A. Reprod Toxicol 59:167–182. https://doi.org/10.1016/j.reprotox.2015.09.006
Rochester JR, Bolden AL (2015) Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Perspect 123(7):643–650. https://doi.org/10.1289/ehp.1408989
European Union (2011) Amending Directive 2002/72/EC as Regards the Restriction of Use of Bisphenol a in Plastic Infant Feeding Bottles, Commission Directive 2011/8/EU of 28 January 2011
US Food and Drug Administration (2013) Amended the regulation 21 CFR175 as regards no longer use bisphenol a in the coating of packaging for powdered and liquid infant formula
Liao C, Liu F, Alomirah H, Loi VD, Mohd MA, Moon H al (2012) Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures. Environ Sci Technol 46:6860–6866
Clark E (2012) Sulfolane and sulfones. Kirk-Othmer encyclopedia of chemical technology. Wiley, New York
Liao C, Li F, Kannan K (2012) Bisphenol S, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environ Sci Technol 46:6515–6522
Atlas E, Dimitrova V (2019) Bisphenol S and bisphenol A disrupt morphogenesis of MCF-12A human mammary epithelial cells. Sci Rep 9(1):1–10
Yamazaki E, Yamashita N, Taniyasu S, Lam J, Lam PK, Moon H al (2015) Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol Environ Saf 122:565–572
Liao CY, Kannan K (2013) Concentrations and Profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for Human Exposure. J Agric Food Chem 61(19):4655–4662
Barboza LGA, Cunha SC, Monteiro C, Fernandes JO, Guilhermino L (2020) Bisphenol A and its analogs in muscle and liver of fish from the North East Atlantic Ocean in relation to microplastic contamination. Exposure and risk to human consumers. J Hazard Mater 393:22419. https://doi.org/10.1016/j.jhazmat.2020.122419
Naderi M, Wong MY, Gholami F (2014) Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquat Toxicol 148:195–203
Frenzilli G, Martorell-Ribera J, Bernardeschi M, Scarcelli V, Jönsson E, Diano N, Moggio M, Guidi P, Sturve J, Asker N (2021) Bisphenol A and bisphenol S induce endocrine and chromosomal alterations in brown brout. Front Endocrinol 12:645519
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf 64(2):178–189
OECD (2019) Test no. 203: fish, acute toxicity test. OECD guidelines for the testing of chemicals, Sect. 2. OECD Publishing, Paris. https://doi.org/10.1787/9789264069961-en
Mortensen AS, Tolfsen CC, Arukwe A (2006) Gene expression patterns in estrogen (nonylphenol) and aryl hydrocarbon receptor agonists (PCB-77) interaction using rainbow trout (Oncorhynchus mykiss) primary hepatocyte culture. J Toxicol Environ Health 69(1-2):1–19. https://doi.org/10.1080/15287390500257792
Feng Y, Jiao Z, Shi J, Li M, Guo Q, Shao B (2016) Effects of bisphenol analogues on steroidogenic gene expression and hormone synthesis in H295R cells. Chemosphere 147:9–19. https://doi.org/10.1016/j.chemosphere.2015.12.081
Kose O, Rachidi W, Beal D, Erkekoglu P, Fayyad-Kazan H, Kocer Gumusel B (2020) The effects of different bisphenol derivatives on oxidative stress, DNA damage and DNA repair in RWPE‐1 cells: A comparative study. J Appl Toxicol 40(5):643–654. https://doi.org/10.1002/jat.3934
Aebi H (1974) Catalase. In: Bergemeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 673–684
Habig WH, Pabst MJ, Jakoby WB (1974) The first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Yilmaz C, Bulus H, Oguztuzun S, Cihan M, Fidan C (2020) The activities of GST isozymes in stomach tissues of female obese patients. Turk J Biochem 45(6):883–889. https://doi.org/10.1515/tjb-2020-0235
Sedlak J, Lindsay RH (1968) Estimation of the total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25(C):192–205. https://doi.org/10.1016/0003-2697(68)90092-4
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods in enzymology, vol 52. Academic Press, Cambridge, pp 302–310
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Hercog K, Maisanaba S, Filipič M, Sollner-Dolenc M, Kač L, Žegura B (2019) Genotoxic activity of bisphenol A and its analogues bisphenol S, bisphenol F and bisphenol AF and their mixtures in human hepatocellular carcinoma (HepG2) cells. Sci Total Environ 687:267–276. https://doi.org/10.1016/j.scitotenv.2019.05.486
Jambor T, Kovacikova E, Greifova H, Kovacik A, Libova L, Lukac N (2019) Assessment of the effective impact of bisphenols on mitochondrial activity and steroidogenesis in a dose-dependency in mice TM3 Leydig cells. Physiol Res 68(4):689–693. https://doi.org/10.33549/physiolres.934200
Russo G, Capuozzo A, Barbato F, Irace C, Santamaria R, Grumetto L (2018) Cytotoxicity of seven bisphenol analogues compared to bisphenol A and relationships with membrane affinity data. Chemosphere 201:432–440. https://doi.org/10.1016/j.chemosphere.2018.03.014
Maćczak A, Cyrkler M, Bukowska B, Michałowicz J (2017) Bisphenol A, bisphenol S, bisphenol F and bisphenol AF induce different oxidative stress and damage in human red blood cells (in vitro study). Toxicol In Vitro 41:143–149. https://doi.org/10.1016/j.tiv.2017.02.018
Modesto KA, Martinez CB (2010) Roundup® causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere 78:294–299. https://doi.org/10.1016/j.chemosphere.2009.10.047
Pigeolet E, Corbisier P, Houbion A, Lambert D, Michiels C, Raes M, Zachary MD, Remacle J (1990) Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech Ageing Dev 51(3):283–297. https://doi.org/10.1016/0047-6374(90)90078-t
Dimitrova MST, Tsinova V, Velcheva V (1994) Combined effect of zinc and lead on the hepatic superoxide dismutase-catalase system in carp (Cyprinus carpio). Biochem Physiol C Toxicol 108:43–46
Zhang R, Liu R, Zong W (2016) Bisphenol S interacts with catalase and induces oxidative stress in mouse liver and renal cells. J Agric Food Chem 64(34):6630–6640
Monteiro DA, Rantin FT, Kalinin AL (2009) The effects of selenium on oxidative stress biomarkers in the freshwater characid fish matrinxã, Brycon cephalus exposed to organophosphate insecticide Folisuper 600 (methyl parathion). Biochem Physiol C Toxicol 149(1):40–49
Aykut H, Kaptaner B (2021) In vitro effects of bisphenol F on antioxidant system indicators in the isolated hepatocytes of rainbow trout (Oncorhyncus mykiss). Mol Biol Rep 48:2591–2599. https://doi.org/10.1007/s11033-021-06310-3
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52(1):711–760
Wu M, Xu H, Shen Y, Qiu W, Yang M (2011) Oxidative stress in zebrafish embryos induced by short-term exposure to bisphenol A, nonylphenol, and their mixture. Environ Toxicol Chem 30(10):2335–2341
Stephensen E, Sturve J, Forlin L (2002) Effects of redox cycling compounds on glutathione content and activity of glutathione-related enzymes in rainbow trout liver. Comp Biochem Physiol C 133(3):435–442. https://doi.org/10.1016/s1532-0456(02)00129-1
Eroglu A, Dogan Z, Kanak EG, Atli G, Canli M (2015) Effects of heavy metals (Cd, Cu, Cr, Pb, Zn) on fish glutathione metabolism. Environ Sci Pollut Res 22(5):3229–3237
Jung JH, Moon YS, Kim BM, Lee YM, Kim M, Rhee JS (2018) Comparative analysis of distinctive transcriptome profiles with biochemical evidence in bisphenol S- and benzo[a]pyrene-exposed liver tissues of the olive flounder Paralichthys olivaceus. PloS One 13(5):e0196425. https://doi.org/10.1371/journal.pone.0196425
Yu IT, Rhee JS, Raisuddin S, Lee JS (2008) Characterization of the glutathione S-transferase-Mu (GSTM) gene sequence and its expression in the hermaphroditic fish, Kryptolebias marmoratus as a function of development, gender type and chemical exposure. Chem Biol Interact 174(2):118–125. https://doi.org/10.1016/j.cbi.2008.05.011
Zare A, Henry D, Chua G, Gordon P, Habibi HR (2018) Differential hepatic gene expression profile of male fathead minnows exposed to daily varying dose of environmental contaminants individually and in mixture. Front Endocrinol 9:749. https://doi.org/10.3389/fendo.2018.00749
Li D, Chen Q, Cao J, Chen H, Li L, Cedergreen N et al (2016) The chronic effects of lignin-derived bisphenol and bisphenol A in Japanese medaka Oryzias latipes. Aquat Toxicol 170:199–207. https://doi.org/10.1016/j.aquatox.2015.11.024
Sandamalika WMG, Priyathilaka TT, Lee S, Yang H, Lee J (2019) Immune and xenobiotic responses of glutathione S-Transferase theta (GST-θ) from marine invertebrate disk abalone (Haliotis discus discus): With molecular characterization and functional analysis. Fish Shellfish Immunol 91:159–171. https://doi.org/10.1016/j.fsi.2019.04.004
Pérez-López M, Anglade P, Bec-Ferté M, Debrauwer L, Perdu E, Cravedi JP, Rouimi P (2000) Characterization of hepatic and extrahepatic glutathione S-transferases in rainbow trout (Oncorhynchus mykiss) and their induction by 3,3′,4,4′-tetrachlorobiphenyl. Fish Physiol Biochem 22:21–32. https://doi.org/10.1023/A:1007885332573
Coggan M, Flanagan JU, Parker MW, Vichai V, Pearson WR, Board PG (2002) Identification and characterization of GSTT3, a third murine Theta class glutathione transferase. Biochem J 366(1):323–332. https://doi.org/10.1042/bj20011878
Yin H, Xu L, Porter NA (2011) Free radical lipid peroxidation: mechanisms and analysis. Chem Rev 111(10):944–5972
Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. https://doi.org/10.1155/2014/360438
Ullah H, Ambreen A, Ahsan N, Jahan S (2017) Bisphenol S induces oxidative stress and DNA damage in rat spermatozoa in vitro and disrupts daily sperm production in vivo. Toxicol Environ Chem 99(5-6):953–965
Ullah H, Jaha S, Ain QU, Shaheen G, Ahsan N (2016) Effect of bisphenol S exposure on male reproductive system of rats: A histological and biochemical study. Chemosphere 152:383–391
Ullah A, Pirzada M, Afsar T, Razak S, Almajwal A, Jahan S (2019) Effect of bisphenol F, an analog of bisphenol A, on the reproductive functions of male rats. Environ Health Prev Med 24(1):41. https://doi.org/10.1186/s12199-019-0797-5
Funding
The authors declare that no financial support was received for either the research or the writing of this article.
Author information
Authors and Affiliations
Contributions
This research was conceived by BK. The isolation and culturing of the hepatocytes, and the application of the treatments were performed by BK, HA, ED, MB and FY. The cytotoxicity testing was performed by BK, HA, ED, and FY. The antioxidant defense system indicators were measured by BK, CY, HA, ED, CF, FY, and MB. Analyzing of the data and interpreting the results were performed by BK and CY. The drafting and editing of the manuscript were performed by BK and CY. The manuscript was finalized by BK.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest with regards to this research.
Ethical approval
The procedures that were conducted within this research were all performed in line with the procedures set forth by the National and Institutional Regulations for the Protection of Animal Welfare. The necessary permissions were obtained from the Ethical Committee of the Animal Experiments Ethics Committee of Van Yuzuncu Yil University, under decision No.: 2021/03-15 and protocol No.: E.37149.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaptaner, B., Yılmaz, C., Aykut, H. et al. Bisphenol S leads to cytotoxicity-induced antioxidant responses and oxidative stress in isolated rainbow trout (Oncorhyncus mykiss) hepatocytes. Mol Biol Rep 48, 7657–7666 (2021). https://doi.org/10.1007/s11033-021-06771-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06771-6