Abstract
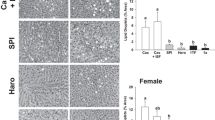
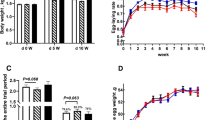
Our previous study showed that soy milks could contain high levels of active soybean trypsin inhibitors (SBTI) if they were not properly processed. This study investigated the effects of consuming active SBTI on pancreatic weights, histology, trypsinogen production and expression of STAT3, receptors for androgen (AR) and estrogen (ER) in pancreas, liver and uterus of rats. Weanling Sprague–Dawley rats were randomly divided into 3 groups (8 females and 8 males/group) and fed diets containing either 20% casein protein (Casein) or 20% soy protein (SP) in the presence of high (1.42 BAEE unit/µg, SP + SBTI) or low (0.2 BAEE unit/µg, SP-SBTI) levels of active SBTI for 8 weeks. Ingestion of SP + SBTI diet markedly increased pancreatic weights and trypsinogen content (p < 0.01), and caused acinar cell hypertrophy, and reduced pancreatic STAT3, p-STAT3, AR and ERβ content, and increased uterine ERα and ERβ compared to the Casein or SP-SBTI diets (p < 0.05). The two SP-containing diets lowered hepatic STAT3, p-STAT3, and pancreatic ERα, and increased hepatic ERα and ERβ content in the female rats compared to the Casein diet (p < 0.05). This study demonstrated for the first time that consumption of high level of active SBTI not only increased pancreatic weights and acinar cell secretions, but also attenuated the expression of pancreatic STAT3, p-STAT3, AR, and ERβ proteins in both sexes and increased uterine ERα and ERβ content, and that dietary soy protein affected hepatic STAT3, p-STAT3, ERα and ERβ in a gender-dependent manner.



Similar content being viewed by others
Abbreviations
- AR:
-
Androgen receptor
- BBI:
-
Bowman-Birk inhibitors
- ER:
-
Estrogen receptor
- KTI:
-
Kunitz trypsin inhibitor
- SBTI:
-
Soybean trypsin inhibitor
- SP:
-
Soy protein
- STAT3:
-
Signal transducer and activator of transcription 3
- p-STAT3:
-
Phosphorylated STAT3
References
Bacon JR, Wanigatunga SCDR, An J, Fenwick GR (1995) A microassay for the analysis of trypsin inhibitor activity in peas. Food Chem 52:77–80
Xu Z, Chen Y, Zhang C, Kong X, Hua Y (2012) The heat-induced protein aggregate correlated with trypsin inhibitor inactivation in soymilk processing. J Agric Food Chem 60:8012–8019
Xiao CW, Wood CM, Robertson P, Gilani GS (2012) Protease inhibitor activities and isoflavone content in commercial soymilks and soy-based infant formulas sold in Ottawa, Canada. J Food Comp Anal 25:130–136
Logsdon CD, Ji B (2013) The role of protein synthesis and digestive enzymes in acinar cell injury. Nat Rev Gastroenterol Hepatol 10:362–370
Yuan S, Chang SK, Liu Z, Xu B (2008) Elimination of trypsin inhibitor activity and beany flavor in soy milk by consecutive blanching and ultrahigh-temperature (UHT) processing. J Agric Food Chem 56:7957–7963
Temler RS, Dormond CA, Simon E, Morel B (1984) The effect of feeding soybean trypsin inhibitor and repeated injections of cholecystokinin on rat pancreas. J Nutr 114:1083–1091
McGuinness EE, Morgan RG, Wormsley KG (1984) Effects of soybean flour on the pancreas of rats. Environ Health Perspect 56:205–212
Cheftel JC, Cuq JL, Lorient D (1985) Amino acids, peptides, and proteins. In: Fennema OR (ed) Food chemistry. Marcel Dekker Inc, New York
Kostromina E, Gustavsson N, Wang X, Lim CY, Radda GK, Li C, Han W (2010) Glucose intolerance and impaired insulin secretion in pancreas-specific signal transducer and activator of transcription-3 knockout mice are associated with microvascular alterations in the pancreas. Endocrinology 151:2050–2059
Xu W, Morford J, Mauvais-Jarvis F (2019) Emerging role of testosterone in pancreatic ß-cell function and insulin secretion. J Endocrinol. https://doi.org/10.1530/JOE-18-0573
Navarro G, Xu W, Jacobson DA, Wicksteed B, Allard C, Zhang G, De GK, Kim SH, Wu H, Zhang H, Verhoeven G, Katzenellenbogen JA, Mauvais-Jarvis F (2016) Extranuclear actions of the androgen receptor enhance glucose-stimulated insulin secretion in the male. Cell Metab 23:837–851
Mishra JS, More AS, Kumar S (2018) Elevated androgen levels induce hyperinsulinemia through increase in Ins1 transcription in pancreatic beta cells in female rats. Biol Reprod 98:520–531
Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquie M, Gauthier BR, Nef S, Stefani E, Nadal A (2008) Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS One 3:e2069
Alonso-Magdalena P, Ropero AB, Garcia-Arevalo M, Soriano S, Quesada I, Muhammed SJ, Salehi A, Gustafsson JA, Nadal A (2013) Antidiabetic actions of an estrogen receptor beta selective agonist. Diabetes 62:2015–2025
Soriano S, Ropero AB, Alonso-Magdalena P, Ripoll C, Quesada I, Gassner B, Kuhn M, Gustafsson JA, Nadal A (2009) Rapid regulation of K(ATP) channel activity by 17ß-estradiol in pancreatic ß-cells involves the estrogen receptor ß and the atrial natriuretic peptide receptor. Mol Endocrinol 23:1973–1982
Vogel H, Mirhashemi F, Liehl B, Taugner F, Kluth O, Kluge R, Joost HG, Schurmann A (2013) Estrogen deficiency aggravates insulin resistance and induces beta-cell loss and diabetes in female New Zealand obese mice. Horm Metab Res 45:430–435
Chen Q, Wood C, Gagnon C, Cober ER, Frégeau-Reid JA, Gleddie S, Xiao CW (2014) The alpha’ subunit of beta-conglycinin and the A1–5 subunits of glycinin are not essential for many hypolipidemic actions of dietary soy proteins in rats. Eur J Nutr 53:1195–1207
AACC (1999) AACC International Method 22-40.01: Measurement of trypsin inhibitor activity of soy products-spectrophotometric method. Approved Methods of Analysis, 11th ed
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Crass RA, Morgan RG (1982) The effect of long-term feeding of soya-bean flour diets on pancreatic growth in the rat. Br J Nutr 47:119–129
Nitsan Z, Nir I, Liener IE (1983) Accentuated response to raw soya-bean meal by meal feeding. Arch Toxicol Suppl 6:177–181
Cheranov SY, Karpurapu M, Wang D, Zhang B, Venema RC, Rao GN (2008) An essential role for SRC-activated STAT-3 in 14,15-EET-induced VEGF expression and angiogenesis. Blood 111:5581–5591
Harada N, Yoda Y, Yotsumoto Y, Masuda T, Takahashi Y, Katsuki T, Kai K, Shiraki N, Inui H, Yamaji R (2018) Androgen signaling expands beta-cell mass in male rats and beta-cell androgen receptor is degraded under high-glucose conditions. Am J Physiol Endocrinol Metab 314:E274–E286
Tiano JP, Delghingaro-Augusto V, Le MC, Liu S, Kaw MK, Khuder SS, Latour MG, Bhatt SA, Korach KS, Najjar SM, Prentki M, Mauvais-Jarvis F (2011) Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents beta cell failure in rodent models of type 2 diabetes. J Clin Invest 121:3331–3342
Coldham NG, Sauer MJ (2000) Pharmacokinetics of [(14)C]Genistein in the rat: gender-related differences, potential mechanisms of biological action, and implications for human health. Toxicol Appl Pharmacol 164:206–215
Xiao CW (2008) Health effects of soy protein and isoflavones in humans. J Nutr 138:1244S-1249S
Thompson C, Lucier GW (1983) Hepatic estrogen responsiveness. Possible mechanisms for sexual dimorphism. Mol Pharmacol 24:69–76
Yamamoto T, Matsuda T, Junicho A, Kishi H, Saatcioglu F, Muraguchi A (2000) Cross-talk between signal transducer and activator of transcription 3 and estrogen receptor signaling. FEBS Lett 486:143–148
Xiao CW, Wood CM, Weber D, Aziz SA, Mehta R, Griffin P, Cockell KA (2014) Dietary supplementation with soy isoflavones or replacement with soy proteins prevents hepatic lipid droplet accumulation and alters expression of genes involved in lipid metabolism in rats. Genes Nutr 9:373
Chatterjee C, Liu J, Wood C, Gagnon C, Cober ER, Fregeau-Reid JA, Gleddie S, Xiao CW (2018) The alpha’ subunit of beta-conglycinin and various glycinin subunits of soy are not required to modulate hepatic lipid metabolism in rats. Eur J Nutr 57:1157–1168
Notides AC, Hamilton DE, Rudolph JH (1973) The action of a human uterine protease on the estrogen receptor. Endocrinology 93:210–216
Tilzer LL, McFarland RT, Plapp FV, Evans JP, Chiga M (1981) Different ionic forms of estrogen receptor in rat uterus and human breast carcinoma. Cancer Res 41:1058–1063
Billings PC, St Clair WH, Maki PA, Kennedy AR (1992) Distribution of the Bowman Birk protease inhibitor in mice following oral administration. Cancer Lett 62:191–197
Finlay TH, Katz J, Rasums A, Seiler S, Levitz M (1981) Estrogen-stimulated uptake of alpha 1-protease inhibitor and other plasma proteins by the mouse uterus. Endocrinology 108:2129–2136
Figarella C, Negri GA, Guy O (1974) Studies on inhibition of the two human trypsins. In: Fritz H, Tschesche H, Greene LJ (eds) Proteinase inhibitors. Proceedings of the 2nd International Research Conference. Springer, Berlin/Heidelberg/New York
Mallory PA, Travis J (1975) Inhibition spectra of the human pancreatic endopeptidases. Am J Clin Nutr 28:823–830
Guerrero-Beltran JA, Estrada-Giron Y, Swanson BG, Barbosa-Canovas GV (2009) Pressure and temperature combination for inactivation of soymilk trypsin inhibitors. Food Chem 116:676–679
Acknowledgements
The authors would like to thank Dr. Stephen Gleddie at Agriculture and Agri-Food Canada for providing soy proteins. We thank the technicians in the Scientific Service Division, Food Directorate, Health Canada for their assistance during the animal experimentation phase.
Funding
This research was supported by Health Canada.
Author information
Authors and Affiliations
Contributions
CWX designed the animal study and conducted data analysis and prepared the manuscript. CW coordidated the animal study and conducted sample analysis. LAC, ML, and MR analysed the samples and participated in manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Informed consent
All authors in this paper have read the final manuscript and approved for publication.
Animal rights
The animal experimental protocol was approved by the Health Canada-Ottawa Animal Care Committee, and all animal handling and care followed the guidelines of the Canadian Council for Animal Care.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiao, CW., Wood, C., Cunningham, L.A. et al. Effects of dietary active soybean trypsin inhibitors on pancreatic weights, histology and expression of STAT3 and receptors for androgen and estrogen in different tissues of rats. Mol Biol Rep 48, 4591–4600 (2021). https://doi.org/10.1007/s11033-021-06491-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06491-x