Abstract
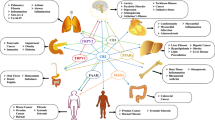
The discovery of endogenous cannabinoid receptors CB1 and CB2 and their endogenous ligands has generated interest in the endocannabinoid system and has contributed to the understanding of the role of the endocannabinoid system. Its role in the normal physiology of the body and its implication in pathological states such as cardiovascular diseases, neoplasm, depression and pain have been subjects of scientific interest. In this review the authors focus on the endogenous cannabinoids, and the critical role of cannabinoid receptor signaling in neurodegeneration and other inflammatory responses such as gut, joint and skin inflammation. This review also discusses the potential of endocannabinoid pathways as drug targets in the amelioration of some inflammatory conditions. Though the exact role of the endocannabinoid system is not fully understood, the evidence found much clearly points to a great potential in exploiting both its central and peripheral pathways in disease management. Cannabinoid therapy has proven promising in several preclinical and clinical trials.
Similar content being viewed by others
References
McCoy KL (2016) Interaction between cannabinoid system and toll-like receptors controls inflammation. Mediators Inflamm 2016:5831315. https://doi.org/10.1155/2016/5831315
Turcotte C, Blanchet MR, Laviolette M, Flamand N (2016) The CB2 receptor and its role as a regulator of inflammation. Cell Mol Life Sci 73(23):4449–4470. https://doi.org/10.1007/s00018-016-2300-4
Munro S, Thomas KL, Abu-Shaar M (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365(6441):61–65. https://doi.org/10.1038/365061a0
Galiègue S, Mary S, Marchand J, Dussossoy D, Carrière D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P (1995) Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem 232(1):54–61. https://doi.org/10.1111/j.1432-1033.1995.tb20780.x
Lu HC, Mackie K (2009) An introduction to the endogenous cannabinoid system. Biol Psychiatry 79(7):516–525. https://doi.org/10.1016/j.biopsych.2015.07.028
Ligresti A, Petrosino S, Di Marzo V (2009) From endocannabinoid profiling to ‘endocannabinoid therapeutics.’ Curr Opin Chem Biol 13(3):321–331. https://doi.org/10.1016/j.cbpa.2009.04.615
Uddin MS, Mamun AA, Sumsuzzman DM, Ashraf GM, Perveen A, Bungau SG, Mousa SA, El-Seedi HR, Bin-Jumah MN, Abdel-Daim MM (2020) Emerging promise of cannabinoids for the management of pain and associated neuropathological alterations in Alzheimer’s disease. Front Pharmacol 11:1097. https://doi.org/10.3389/fphar.2020.01097
Zeng J, Li X, Cheng Y, Ke B, Wang R (2019) Activation of cannabinoid receptor type 2 reduces lung ischemia reperfusion injury through PI3K/Akt pathway. Int J Clin Exp Pathol 12(11):4096–4105
Zhao Y, Yuan Z, Liu Y, Xue J, Tian Y, Liu W, Zhang W, Shen Y, Xu W, Liang X, Chen T (2010) Activation of cannabinoid CB2 receptor ameliorates atherosclerosis associated with suppression of adhesion molecules. J Cardiovasc Pharmacol 55(3):292–298. https://doi.org/10.1097/FJC.0b013e3181d2644d
Argenziano M, Tortora C, Bellini G, Di Paola A, Punzo F, Rossi F (2019) The endocannabinoid system in pediatric inflammatory and immune diseases. Int J Mol Sci 20(23):5875. https://doi.org/10.3390/ijms20235875.Erratum.In:IntJMolSci21(8) (PMID: 31771129)
Bie B, Wu J, Foss JF, Naguib M (2018) An overview of the cannabinoid type 2 receptor system and its therapeutic potential. Curr Opin Anaesthesiol 31(4):407–414. https://doi.org/10.1097/ACO.0000000000000616
Barrie N, Manolios N (2017) The endocannabinoid system in pain and inflammation: its relevance to rheumatic disease. Eur J Rheumatol 4(3):210–218. https://doi.org/10.5152/eurjrheum.2017.17025
Katona I, Freund TF (2012) Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci 35:529–558. https://doi.org/10.1146/annurev-neuro-062111-150420
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258(5090):1946–1949. https://doi.org/10.1126/science.1470919
Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR et al (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50(1):83–90. https://doi.org/10.1016/0006-2952(95)00109-d
Monteleone AM, Di Marzo V, Aveta T, Piscitelli F, Dalle Grave R, Scognamiglio P, El Ghoch M, Calugi S, Monteleone P, Maj M (2015) Deranged endocannabinoid responses to hedonic eating in underweight and recently weight-restored patients with anorexia nervosa. Am J Clin Nutr 101(2):262–269. https://doi.org/10.3945/ajcn.114.096164
Sugiura T, Kondo S, Kishimoto S, Miyashita T, Nakane S, Kodaka T, Suhara Y, Takayama H, Waku K (2000) Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. J Biol Chem 275(1):605–12. https://doi.org/10.1074/jbc.275.1.605
Sharkey KA, Wiley JW (2016) The role of the endocannabinoid system in the brain–gut axis. Gastroenterology 151(2):252–266. https://doi.org/10.1053/j.gastro.2016.04.015
Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D (1994) Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 372(6507):686–691. https://doi.org/10.1038/372686a0
Simon GM, Cravatt BF (2006) Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J Biol Chem 281(36):26465–26472. https://doi.org/10.1074/jbc.M604660200
Simon GM, Cravatt BF (2008) Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acyl ethanolamine precursors in mouse brain. J Biol Chem 283(14):9341–9349. https://doi.org/10.1074/jbc.M707807200
Blankman JL, Cravatt BF (2013) Chemical probes of endocannabinoid metabolism. Pharmacol Rev 65(2):849–871. https://doi.org/10.1124/pr.112.006387
Stella N, Schweitzer P, Piomelli D (1997) A second endogenous cannabinoid that modulates long-term potentiation. Nature 388(6644):773–778. https://doi.org/10.1038/42015
Min R, Di Marzo V, Mansvelder HD (2010) DAG lipase involvement in depolarization-induced suppression of inhibition: does endocannabinoid biosynthesis always meet the demand? Neuroscientist 16(6):608–613. https://doi.org/10.1177/1073858410373281
Alger BE, Kim J (2011) Supply and demand for endocannabinoids. Trends Neurosci 34(6):304–315. https://doi.org/10.1016/j.tins.2011.03.003
Burstein SH, Zurier RB (2009) Cannabinoids, endocannabinoids, and related analogs in inflammation. AAPS J 11(1):109–119. https://doi.org/10.1208/s12248-009-9084-5
Blankman JL, Simon GM, Cravatt BF (2007) A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol 14(12):1347–1356. https://doi.org/10.1016/j.chembiol.2007.11.006
Hermanson DJ, Gamble-George JC, Marnett LJ, Patel S (2014) Substrate-selective COX-2 inhibition as a novel strategy for therapeutic endocannabinoid augmentation. Trends Pharmacol Sci 35(7):358–367. https://doi.org/10.1016/j.tips.2014.04.006
Alhouayek M, Muccioli GG (2014) COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol Sci 35(6):284–292. https://doi.org/10.1016/j.tips.2014.03.001
Wang J, Ueda N (2009) Biology of endocannabinoid synthesis system. Prostaglandins Other Lipid Mediat 89(3–4):112–119. https://doi.org/10.1016/j.prostaglandins.2008.12.002
Martin WJ, Patrick SL, Coffin PO, Tsou K, Walker JM (1995) An examination of the central sites of action of cannabinoid-induced antinociception in the rat. Life Sci 56(23–24):2103–2109. https://doi.org/10.1016/0024-3205(95)00195-c
Trautmann SM, Sharkey KA (2015) The endocannabinoid system and its role in regulating the intrinsic neural circuitry of the gastrointestinal tract. Int Rev Neurobiol 125:85–126. https://doi.org/10.1016/bs.irn.2015.10.002
Wright KL, Duncan M, Sharkey KA (2008) Cannabinoid CB2 receptors in the gastrointestinal tract: a regulatory system in states of inflammation. Br J Pharmacol 153(2):263–270. https://doi.org/10.1038/sj.bjp.0707486
Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, Vanderah TW, Lai J, Porreca F, Makriyannis A, Malan TP Jr (2003) Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci USA 100(18):10529–10533. https://doi.org/10.1073/pnas.1834309100
Donvito G, Nass SR, Wilkerson JL, Curry ZA, Schurman LD, Kinsey SG, Lichtman AH (2018) The endogenous cannabinoid system: a budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology 43(1):52–79. https://doi.org/10.1038/npp.2017.204
Klein TW (2005) Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol 5(5):400–411. https://doi.org/10.1038/nri1602
Cabral GA, Griffin-Thomas L (2009) Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert Rev Mol Med 11:e3. https://doi.org/10.1017/S1462399409000957
Sancho R, Calzado MA, Di Marzo V, Appendino G, Muñoz E (2003) Anandamide inhibits nuclear factor-kappaB activation through a cannabinoid receptor-independent pathway. Mol Pharmacol 63(2):429–438. https://doi.org/10.1124/mol.63.2.429
Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, Hoertnagl H, Raine CS, Schneider-Stock R, Nitsch R, Ullrich O (2006) The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron 49(1):67–79. https://doi.org/10.1016/j.neuron.2005.11.027
Klein TW, Newton CA, Widen R, Friedman H (1985) The effect of delta-9-tetrahydrocannabinol and 11-hydroxy-delta-9-tetrahydrocannabinol on T-lymphocyte and B-lymphocyte mitogen responses. J Immunopharmacol 7(4):451–466. https://doi.org/10.3109/08923978509026487
Berdyshev EV, Boichot E, Germain N, Allain N, Anger JP, Lagente V (1997) Influence of fatty acid ethanolamides and delta9-tetrahydrocannabinol on cytokine and arachidonate release by mononuclear cells. Eur J Pharmacol 330(2–3):231–240. https://doi.org/10.1016/s0014-2999(97)01007-8
Chang YH, Lee ST, Lin WW (2001) Effects of cannabinoids on LPS-stimulated inflammatory mediator release from macrophages: involvement of eicosanoids. J Cell Biochem 81(4):715–723. https://doi.org/10.1002/jcb.1103
Yuan M, Kiertscher SM, Cheng Q, Zoumalan R, Tashkin DP, Roth MD (2002) Delta 9-Tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J Neuroimmunol 133(1–2):124–131. https://doi.org/10.1016/s0165-5728(02)00370-3
Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M (2009) Cannabinoids as novel anti-inflammatory drugs. Future Med Chem 1(7):1333–1349. https://doi.org/10.4155/fmc.09.93
Sacerdote P, Massi P, Panerai AE, Parolaro D (2000) In vivo and in vitro treatment with the synthetic cannabinoid CP55, 940 decreases the in vitro migration of macrophages in the rat: involvement of both CB1 and CB2 receptors. J Neuroimmunol 109(2):155–163. https://doi.org/10.1016/S0165-5728(00)00307-6
Klein TW, Cabral GA (2006) Cannabinoid-induced immune suppression and modulation of antigen-presenting cells. J Neuroimmune Pharmacol 1(1):50–64. https://doi.org/10.1007/s11481-005-9007-x
McKallip RJ, Lombard C, Martin BR, Nagarkatti M, Nagarkatti PS (2002) Delta(9)-tetrahydrocannabinol-induced apoptosis in the thymus and spleen as a mechanism of immunosuppression in vitro and in vivo. J Pharmacol Exp Ther 302(2):451–465. https://doi.org/10.1124/jpet.102.033506
Romero-Sandoval EA, Horvath RJ, DeLeo JA (2008) Neuroimmune interactions and pain: focus on glial-modulating targets. Curr Opin Investig Drugs 9(7):726–34 (PMID: 18600578; PMCID: PMC2696046)
Villacampa N, Heneka MT (2018) Microglia: you’ll never walk alone! Immunity 48(2):195–197. https://doi.org/10.1016/j.immuni.2018.02.009
Schwartz M, Butovsky O, Brück W, Hanisch UK (2006) Microglial phenotype: is the commitment reversible? Trends Neurosci 29(2):68–74. https://doi.org/10.1016/j.tins.2005.12.005
Schwartz M, Shaked I, Fisher J, Mizrahi T, Schori H (2003) Protective autoimmunity against the enemy within: fighting glutamate toxicity. Trends Neurosci 26(6):297–302. https://doi.org/10.1016/S0166-2236(03)00126-7
Ashton JC, Glass M (2007) The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr Neuropharmacol 5(2):73–80. https://doi.org/10.2174/157015907780866884
Zipp F, Aktas O (2006) The brain as a target of inflammation: common pathways link inflammatory and neurodegenerative diseases. Trends Neurosci 29(9):518–527. https://doi.org/10.1016/j.tins.2006.07.006
Tremblay MÈ, Majewska AK (2011) A role for microglia in synaptic plasticity? Commun Integr Biol 4(2):220–222. https://doi.org/10.4161/cib.4.2.14506
Schafer DP, Stevens B (2013) Phagocytic glial cells: sculpting synaptic circuits in the developing nervous system. Curr Opin Neurobiol 23(6):1034–1040. https://doi.org/10.1016/j.conb.2013.09.012
Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT (2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333(6048):1456–1458. https://doi.org/10.1126/science.1202529
Wake H, Moorhouse AJ, Miyamoto A, Nabekura J (2013) Microglia: actively surveying and shaping neuronal circuit structure and function. Trends Neurosci 36(4):209–217. https://doi.org/10.1016/j.tins.2012.11.007
Naguib M, Xu JJ, Diaz P, Brown DL, Cogdell D, Bie B, Hu J, Craig S, Hittelman WN (2012) Prevention of paclitaxel-induced neuropathy through activation of the central cannabinoid type 2 receptor system. Anesth Analg 114(5):1104–1120. https://doi.org/10.1213/ANE.0b013e31824b0191
Ramírez BG, Blázquez C, Gómez del Pulgar T, Guzmán M, de Ceballos ML (2005) Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci 25(8):1904–1913. https://doi.org/10.1523/JNEUROSCI.4540-04.2005
Benito C, Tolón RM, Castillo AI, Ruiz-Valdepenas L, Martinez-Orgado JA, Fernandez-Sanchez FJ, Vazquez C, Cravatt BF, Romero J (2012) β-Amyloid exacerbates inflammation in astrocytes lacking fatty acid amide hydrolase through a mechanism involving PPAR-α, PPAR-γ and TRPV1, but not CB1 or CB2 receptors. Br J Pharmacol 166(4):1474–1489. https://doi.org/10.1111/j.1476-5381.2012.01889.x
Jin K, Xie L, Kim SH, Parmentier-Batteur S, Sun Y, Mao XO, Childs J, Greenberg DA (2004) Defective adult neurogenesis in CB1 cannabinoid receptor knockout mice. Mol Pharmacol 66(2):204–208. https://doi.org/10.1124/mol.66.2.204
Cabral GA, Marciano-Cabral F (2005) Cannabinoid receptors in microglia of the central nervous system: immune functional relevance. J Leukoc Biol 78(6):1192–1197. https://doi.org/10.1189/jlb.0405216
Ehrhart J, Obregon D, Mori T, Hou H, Sun N, Bai Y, Klein T, Fernandez F, Tan J, Shytle RD (2005) Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J Neuroinflammation 2:29. https://doi.org/10.1186/1742-2094-2-29
Rossi S, Bozzali M, Bari M, Mori F, Studer V, Motta C, Buttari F, Cercignani M, Gravina P, Mastrangelo N, Castelli M, Mancino R, Nucci C, Sottile F, Bernardini S, Maccarrone M, Centonze D (2013) Association between a genetic variant of type-1 cannabinoid receptor and inflammatory neurodegeneration in multiple sclerosis. PLoS One 8(12):e82848. https://doi.org/10.1371/journal.pone.0082848
Cassano T, Calcagnini S, Pace L, De Marco F, Romano A, Gaetani S (2017) Cannabinoid receptor 2 signaling in neurodegenerative disorders: from pathogenesis to a promising therapeutic target. Front Neurosci 11:30. https://doi.org/10.3389/fnins.2017.00030
Grill M, Hasenoehrl C, Storr M, Schicho R (2018) Medical cannabis and cannabinoids: an option for the treatment of inflammatory bowel disease and cancer of the colon? Med Cannabis Cannabinoids 1(1):28–35. https://doi.org/10.1159/000489036
Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K (2008) GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci U S A 105(7):2699–2704. https://doi.org/10.1073/pnas.0711278105
Sibaev A, Yüce B, Kemmer M, Nassauw LV, Broedl U, Allescher HD, Göke B, Timmermans JP, Storr M (2009) Cannabinoid-1 (CB1) receptors regulate colonic propulsion by acting at motor neurons within the ascending motor pathways in mouse colon. Am J Physiol-Gastrointestinal Liver Physiol 296(1):G119–G128. https://doi.org/10.1152/ajpgi.90274.2008
Schmöle AC, Lundt R, Gennequin B et al (2015) Correction: expression analysis of CB2-GFP BAC transgenic mice. PLoS One 10(12):e0145472. https://doi.org/10.1371/journal.pone.0145472
Cani PD, Plovier H, Van Hul M, Geurts L, Delzenne NM, Druart C, Everard A (2016) Endocannabinoids–at the crossroads between the gut microbiota and host metabolism. Nat Rev Endocrinol 12(3):133–143. https://doi.org/10.1038/nrendo.2015.211
Karwad MA, Couch DG, Theophilidou E, Sarmad S, Barrett DA, Larvin M, Wright KL, Lund JN, O’Sullivan SE (2017) The role of CB1 in intestinal permeability and inflammation. FASEB J 31(8):3267–3277. https://doi.org/10.1096/fj.201601346R
Acharya N, Penukonda S, Shcheglova T, Hagymasi AT, Basu S, Srivastava PK (2017) Endocannabinoid system acts as a regulator of immune homeostasis in the gut. PNAS 114(19):5005–5010. https://doi.org/10.1073/pnas.1612177114
Di Sabatino A, Battista N, Biancheri P, Rapino C, Rovedatti L, Astarita G, Vanoli A, Dainese E, Guerci M, Piomelli D, Pender SL, MacDonald TT, Maccarrone M, Corazza GR (2011) The endogenous cannabinoid system in the gut of patients with inflammatory bowel disease. Mucosal Immunol 4(5):574–583. https://doi.org/10.1038/mi.2011.18
Chen L, Chen H, Li Y, Li L, Qiu Y, Ren J (2015) Endocannabinoid and ceramide levels are altered in patients with colorectal cancer. Oncol Rep 34(1):447–454. https://doi.org/10.3892/or.2015.3973
Massa F, Marsicano G, Hermann H, Cannich A, Monory K, Cravatt BF, Ferri GL, Sibaev A, Storr M, Lutz B (2004) The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest 113(8):1202–1209. https://doi.org/10.1172/JCI19465
D’Argenio G, Valenti M, Scaglione G, Cosenza V, Sorrentini I, Di Marzo V (2006) Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J 20(3):568–570. https://doi.org/10.1096/fj.05-4943fje
Kimball ES, Schneider CR, Wallace NH, Hornby PJ (2006) Agonists of cannabinoid receptor 1 and 2 inhibit experimental colitis induced by oil of mustard and by dextran sulfate sodium. Am J Physiol Gastrointest Liver Physiol 291(2):G364–G371. https://doi.org/10.1152/ajpgi.00407.2005
Storr MA, Keenan CM, Zhang H, Patel KD, Makriyannis A, Sharkey KA (2009) Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm Bowel Dis 15(11):1678–1685. https://doi.org/10.1002/ibd.20960
Storr MA, Keenan CM, Emmerdinger D, Zhang H, Yüce B, Sibaev A, Massa F, Buckley NE, Lutz B, Göke B, Brand S, Patel KD, Sharkey KA (2008) Targeting endocannabinoid degradation protects against experimental colitis in mice: involvement of CB1 and CB2 receptors. J Mol Med (Berl) 86(8):925–936. https://doi.org/10.1007/s00109-008-0359-6
Pagano E, Capasso R, Piscitelli F, Romano B, Parisi OA, Finizio S, Lauritano A, Marzo VD, Izzo AA, Borrelli F (2016) An orally active cannabis extract with high content in cannabidiol attenuates chemically-induced intestinal inflammation and hypermotility in the mouse. Front Pharmacol 7:341. https://doi.org/10.3389/fphar.2016.00341
Shamran H, Singh NP, Zumbrun EE et al (2017) Fatty acid amide hydrolase (FAAH) blockade ameliorates experimental colitis by altering microRNA expression and suppressing inflammation. Brain Behav Immun 59:10–20. https://doi.org/10.1016/j.bbi.2016.06.008
Zhao X, Liang P, Liu J, Jiang H, Fan X, Chen G, Zhou C (2017) Elevation of arachidonoylethanolamide levels by activation of the endocannabinoid system protects against colitis and ameliorates remote organ lesions in mice. Exp Ther Med 14(6):5664–5670. https://doi.org/10.3892/etm.2017.5222
Jamontt JM, Molleman A, Pertwee RG, Parsons ME (2010) The effects of Delta-tetrahydrocannabinol and cannabidiol alone and in combination on damage, inflammation and in vitro motility disturbances in rat colitis. Br J Pharmacol 160(3):712–723. https://doi.org/10.1111/j.1476-5381.2010.00791.x
Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ (2007) The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol 152(7):1092–1101. https://doi.org/10.1038/sj.bjp.0707460
De Filippis D, Esposito G, Cirillo C, Cipriano M, De Winter BY, Scuderi C, Sarnelli G, Cuomo R, Steardo L, De Man JG, Iuvone T (2011) Cannabidiol reduces intestinal inflammation through the control of neuroimmune axis. PLoS ONE 6(12):e28159. https://doi.org/10.1371/journal.pone.0028159
De Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, Stott CG, Di Marzo V (2011) Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol 163(7):1479–1494. https://doi.org/10.1111/j.1476-5381.2010.01166.x
Borrelli F, Aviello G, Romano B, Orlando P, Capasso R, Maiello F, Guadagno F, Petrosino S, Capasso F, Di Marzo V, Izzo AA (2009) Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J Mol Med (Berl) 87(11):1111–1121. https://doi.org/10.1007/s00109-009-0512-x
Schicho R, Bashashati M, Bawa M, McHugh D, Saur D, Hu HM, Zimmer A, Lutz B, Mackie K, Bradshaw HB, McCafferty DM, Sharkey KA, Storr M (2011) The atypical cannabinoid O-1602 protects against experimental colitis and inhibits neutrophil recruitment. Inflamm Bowel Dis 17(8):1651–1664. https://doi.org/10.1002/ibd.21538
Leinwand KL, Jones AA, Huang RH, Jedlicka P, Kao DJ, de Zoeten EF, Ghosh S, Moaddel R, Wehkamp J, Ostaff MJ, Bader J, Aherne CM, Collins CB (2017) Cannabinoid receptor-2 ameliorates inflammation in murine model of crohn’s disease. J Crohns Colitis 11(11):1369–1380. https://doi.org/10.1093/ecco-jcc/jjx096
Soethoudt M, Grether U, Fingerle J et al (2017) Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat Commun 8:13958. https://doi.org/10.1038/ncomms13958
Borrelli F, Fasolino I, Romano B, Capasso R, Maiello F, Coppola D, Orlando P, Battista G, Pagano E, Di Marzo V, Izzo AA (2013) Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem Pharmacol 85(9):1306–1316. https://doi.org/10.1016/j.bcp.2013.01.017
Romano B, Borrelli F, Fasolino I, Capasso R, Piscitelli F, Cascio M, Pertwee R, Coppola D, Vassallo L, Orlando P, Di Marzo V, Izzo A (2013) The cannabinoid TRPA1 agonist cannabichromene inhibits nitric oxide production in macrophages and ameliorates murine colitis. Br J Pharmacol 169(1):213–229. https://doi.org/10.1111/bph.12120
Feng YJ, Li YY, Lin XH, Li K, Cao MH (2016) Anti-inflammatory effect of cannabinoid agonist WIN55, 212 on mouse experimental colitis is related to inhibition of p38MAPK. World J Gastroenterol 22(43):9515–9524. https://doi.org/10.3748/wjg.v22.i43.9515
Lin S, Li Y, Shen L, Zhang R, Yang L, Li M, Li K, Fichna J (2017) The anti-inflammatory effect and intestinal barrier protection of HU210 differentially depend on TLR4 signaling in dextran sulfate sodium-induced murine colitis. Dig Dis Sci 62(2):372–386. https://doi.org/10.1007/s10620-016-4404-y
Borrelli F, Romano B, Petrosino S, Pagano E, Capasso R, Coppola D, Battista G, Orlando P, Di Marzo V, Izzo AA (2015) Palmitoylethanolamide, a naturally occurring lipid, is an orally effective intestinal anti-inflammatory agent. Br J Pharmacol 172(1):142–158. https://doi.org/10.1111/bph.12907
Rosa AC, Fantozzi R (2013) The role of histamine in neurogenic inflammation. Br J Pharmacol 170(1):38–45. https://doi.org/10.1111/bph.12266
Bánvölgyi A, Pozsgai G, Brain SD, Helyes ZS, Szolcsányi J, Ghosh M, Melegh B, Pintér E (2004) Mustard oil induces a transient receptor potential vanilloid 1 receptor-independent neurogenic inflammation and a non-neurogenic cellular inflammatory component in mice. Neuroscience 125(2):449–459. https://doi.org/10.1016/j.neuroscience.2004.01.009
Heppelmann B, Pawlak M (1997) Sensitisation of articular afferents in normal and inflamed knee joints by substance P in the rat. Neurosci Lett 223(2):97–100. https://doi.org/10.1016/S0304-3940(97)13408-5
Raychaudhuri SP, Raychaudhuri SK, Atkuri KR, Herzenberg LA, Herzenberg LA (2011) Nerve growth factor: a key local regulator in the pathogenesis of inflammatory arthritis. Arthritis Rheum 63(11):3243–3252. https://doi.org/10.1002/art.30564
Mlost J, Kostrzewa M, Malek N, Starowicz K (2018) Molecular understanding of the activation of CB1 and blockade of TRPV1 receptors: implications for novel treatment strategies in osteoarthritis. Int J Mol Sci 19(2):342. https://doi.org/10.3390/ijms19020342
Ahluwalia J, Urban L, Bevan S, Nagy I (2003) Anandamide regulates neuropeptide release from capsaicin-sensitive primary sensory neurons by activating both the cannabinoid 1 receptor and the vanilloid receptor 1 in vitro. Eur J Neurosci 17(12):2611–2618. https://doi.org/10.1046/j.1460-9568.2003.02703.x
Starowicz K, Makuch W, Korostynski M, Malek N, Slezak M, Zychowska M, Petrosino S, De Petrocellis L, Cristino L, Przewlocka B, Di Marzo V (2013) Full inhibition of spinal FAAH leads to TRPV1-mediated analgesic effects in neuropathic rats and possible lipoxygenase-mediated remodeling of anandamide metabolism. PLoS One 8(4):e60040. https://doi.org/10.1371/journal.pone.0060040
Hermann H, De Petrocellis L, Bisogno T, Schiano Moriello A, Lutz B, Di Marzo V (2003) Dual effect of cannabinoid CB1 receptor stimulation on a vanilloid VR1 receptor-mediated response. Cell Mol Life Sci 60(3):607–616. https://doi.org/10.1007/s000180300052
Maione S, Bisogno T, de Novellis V, Palazzo E, Cristino L, Valenti M, Petrosino S, Guglielmotti V, Rossi F, Di Marzo V (2006) Elevation of endocannabinoid levels in the ventrolateral periaqueductal grey through inhibition of fatty acid amide hydrolase affects descending nociceptive pathways via both cannabinoid receptor type 1 and transient receptor potential vanilloid type-1 receptors. J Pharmacol Exp Ther 316(3):969–982. https://doi.org/10.1124/jpet.105.093286
Scheau C, Badarau IA, Mihai LG, Scheau AE, Costache DO, Constantin C, Calina D, Caruntu C, Costache RS, Caruntu A (2020) Cannabinoids in the pathophysiology of skin inflammation. Molecules 25(3):652. https://doi.org/10.3390/molecules25030652
Sanclemente G, Burgos C, Nova J, Hernández F, González C, Reyes MI, Córdoba N, Arévalo Á, Meléndez E, Colmenares J, Ariza S, Hernández G, Asociación Colombiana de Dermatología y Cirugía Dermatológica (Asocolderma) (2017) The impact of skin diseases on quality of life: a multicenter study. Actas Dermosifiliogr 108(3):244–252. https://doi.org/10.1016/j.ad.2016.11.008 (English, Spanish)
Chovatiya R, Silverberg JI (2019) Pathophysiology of atopic dermatitis and psoriasis: implications for management in children. Children (Basel) 6(10):108. https://doi.org/10.3390/children6100108
Huestis MA (2007) Human cannabinoid pharmacokinetics. Chem Biodivers 4(8):1770–1804. https://doi.org/10.1002/cbdv.200790152
Kaplan DH, Igyártó BZ, Gaspari AA (2012) Early immune events in the induction of allergic contact dermatitis. Nat Rev Immunol 12(2):114–124. https://doi.org/10.1038/nri3150
Novak-Bilić G, Vučić M, Japundžić I, Meštrović-Štefekov J, Stanić-Duktaj S, Lugović-Mihić L (2018) Irritant and allergic contact dermatitis - skin lesion characteristics. Acta Clin Croat 57(4):713–720. https://doi.org/10.20471/acc.2018.57.04.13
Petrosino S, Verde R, Vaia M, Allarà M, Iuvone T, Di Marzo V (2018) Anti-inflammatory properties of cannabidiol, a nonpsychotropic cannabinoid, in experimental allergic contact dermatitis. J Pharmacol Exp Ther 365(3):652–663. https://doi.org/10.1124/jpet.117.244368
Kozela E, Juknat A, Kaushansky N, Ben-Nun A, Coppola G, Vogel Z (2015) Cannabidiol, a non-psychoactive cannabinoid, leads to EGR2-dependent anergy in activated encephalitogenic T cells. J Neuroinflammation 12:52. https://doi.org/10.1186/s12974-015-0273-0
Harvey BS, Sia TC, Wattchow DA, Smid SD (2014) Interleukin 17A evoked mucosal damage is attenuated by cannabidiol and anandamide in a human colonic explant model. Cytokine 65(2):236–244. https://doi.org/10.1016/j.cyto.2013.10.006
Kim HJ, Kim B, Park BM, Jeon JE, Lee SH, Mann S, Ahn SK, Hong SP, Jeong SK (2015) Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int J Dermatol 54(10):e401–e408. https://doi.org/10.1111/ijd.12841
Gaffal E, Cron M, Glodde N, Tüting T (2013) Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy 68(8):994–1000. https://doi.org/10.1111/all.12183
Brancato SK, Albina JE (2011) Wound macrophages as key regulators of repair: origin, phenotype, and function. Am J Pathol 178(1):19–25. https://doi.org/10.1016/j.ajpath.2010.08.003
Arango Duque G, Descoteaux A (2014) Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol 5:491. https://doi.org/10.3389/fimmu.2014.00491
Luo XQ, Li A, Yang X, Xiao X, Hu R, Wang TW, Dou XY, Yang DJ, Dong Z (2018) Paeoniflorin exerts neuroprotective effects by modulating the M1/M2 subset polarization of microglia/macrophages in the hippocampal CA1 region of vascular dementia rats via cannabinoid receptor 2. Chin Med 13:14. https://doi.org/10.1186/s13020-018-0173-1
Li SS, Wang LL, Liu M, Jiang SK, Zhang M, Tian ZL, Wang M, Li JY, Zhao R, Guan DW (2016) Cannabinoid CB2 receptors are involved in the regulation of fibrogenesis during skin wound repair in mice. Mol Med Rep 13(4):3441–3450. https://doi.org/10.3892/mmr.2016.4961
Wang LL, Zhao R, Li JY, Li SS, Liu M, Wang M, Zhang MZ, Dong WW, Jiang SK, Zhang M, Tian ZL, Liu CS, Guan DW (2016) Pharmacological activation of cannabinoid 2 receptor attenuates inflammation, fibrogenesis, and promotes re-epithelialization during skin wound healing. Eur J Pharmacol 786:128–136. https://doi.org/10.1016/j.ejphar.2016.06.006
Tang M, Cao X, Zhang K et al (2018) Celastrol alleviates renal fibrosis by upregulating cannabinoid receptor 2 expression. Cell Death Dis 9(6):1–12
He C, Ryan AJ, Murthy S, Carter AB (2013) Accelerated development of pulmonary fibrosis via Cu, Zn-superoxide dismutase-induced alternative activation of macrophages. J Biol Chem 288(28):20745–20757. https://doi.org/10.1074/jbc.M112.410720
Duru N, Wolfson B, Zhou Q (2016) Mechanisms of the alternative activation of macrophages and non-coding RNAs in the development of radiation-induced lung fibrosis. World J Biol Chem 7(4):231–239. https://doi.org/10.4331/wjbc.v7.i4.231
Braun M, Khan ZT, Khan MB, Kumar M, Ward A, Achyut BR, Arbab AS, Hess DC, Hoda MN, Baban B, Dhandapani KM, Vaibhav K (2018) Selective activation of cannabinoid receptor-2 reduces neuroinflammation after traumatic brain injury via alternative macrophage polarization. Brain Behav Immun 68:224–237. https://doi.org/10.1016/j.bbi.2017.10.021
Sheriff T, Lin MJ, Dubin D, Khorasani H (2020) The potential role of cannabinoids in dermatology. J Dermatolog Treat 31(8):839–845. https://doi.org/10.1080/09546634.2019.1675854
Wilkinson JD, Williamson EM (2007) Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J Dermatol Sci 45(2):87–92. https://doi.org/10.1016/j.jdermsci.2006.10.009
Ramot Y, Sugawara K, Zákány N, Tóth BI, Bíró T, Paus R (2013) A novel control of human keratin expression: cannabinoid receptor 1-mediated signaling down-regulates the expression of keratins K6 and K16 in human keratinocytes in vitro and in situ. PeerJ 1:e40. https://doi.org/10.7717/peerj.40
Changoer L, Anastassov G (2019) Method to treat psoriasis US Patent Application No. 16/106,420.
Du AX, Osman M, Gniadecki R (2020) Use of extracorporeal photopheresis in scleroderma: a review. Dermatology 236(2):105–110. https://doi.org/10.1159/000501591
del Río C, Navarrete C, Collado JA, Bellido ML, Gómez-Cañas M, Pazos MR, Fernández-Ruiz J, Pollastro F, Appendino G, Calzado MA, Cantarero I, Muñoz E (2016) The cannabinoid quinol VCE-004.8 alleviates bleomycin-induced scleroderma and exerts potent antifibrotic effects through peroxisome proliferator-activated receptor-γ and CB2 pathways. Sci Rep 6:21703. https://doi.org/10.1038/srep21703
Burstein SH (2018) Ajulemic acid: potential treatment for chronic inflammation. Pharmacol Res Perspect 6(2):e00394. https://doi.org/10.1002/prp2.394
Man A, Correa JK, Ziemek J, Simms RW, Felson DT, Lafyatis R (2017) Development and validation of a patient-reported outcome instrument for skin involvement in patients with systemic sclerosis. Ann Rheum Dis 76(8):1374–1380. https://doi.org/10.1136/annrheumdis-2016-210534
Spiera RF, Hummers LK, Chung L, et al (2017) A phase 2 study of safety and efficacy of anabasum (JBT-101), a cannabinoid receptor type 2 agonist, in diffuse cutaneous systemic sclerosis. Arthritis & Rheumatology 69.
Martyanov V, Nesbeth Y, Cai G, et al (2017) Effect of anabasum (JBT-101) on gene expression in skin biopsies from subjects with diffuse cutaneous systemic sclerosis (dcSSc) and the relationship of baseline molecular subsets to clinical benefit in the phase 2 trial. Arthritis & Rheumatology 69.
Ali A, Akhtar N (2015) The safety and efficacy of 3% Cannabis seeds extract cream for reduction of human cheek skin sebum and erythema content. Pak J Pharm Sci 28(4):1389–1395
Dobrosi N, Tóth BI, Nagy G, Dózsa A, Géczy T, Nagy L, Zouboulis CC, Paus R, Kovács L, Bíró T (2008) Endocannabinoids enhance lipid synthesis and apoptosis of human sebocytes via cannabinoid receptor-2-mediated signaling. FASEB J 22(10):3685–3695. https://doi.org/10.1096/fj.07-104877
Oláh A, Markovics A, Szabó-Papp J, Szabó PT, Stott C, Zouboulis CC, Bíró T (2016) Differential effectiveness of selected non-psychotropic phytocannabinoids on human sebocyte functions implicates their introduction in dry/seborrhoeic skin and acne treatment. Exp Dermatol 25(9):701–707. https://doi.org/10.1111/exd.13042
Oláh A, Tóth BI, Borbíró I, Sugawara K, Szöllõsi AG, Czifra G, Pál B, Ambrus L, Kloepper J, Camera E, Ludovici M, Picardo M, Voets T, Zouboulis CC, Paus R, Bíró T (2014) Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J Clin Invest 124(9):3713–3724. https://doi.org/10.1172/JCI64628
De Petrocellis L, Orlando P, Moriello AS, Aviello G, Stott C, Izzo AA, Di Marzo V (2012) Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol (Oxf) 204(2):255–266. https://doi.org/10.1111/j.1748-1716.2011.02338.x
Volc-Platzer B (2015) Dermatomyositis - update [dermatomyositis-update]. Hautarzt 66(8):604–10. https://doi.org/10.1007/s00105-015-3659-0 (German)
Chen K, Zeidi M, Reddy N, White B, Werth V (2019) FRI0307 Lenabasum, A cannabinoid type 2 receptor agonist, reduces CD4 cell populations and downregulates type 1 and 2 interferon activities in lesional dermatomyositis skin. Ann Rheum Dis 78(2):835. https://doi.org/10.1136/annrheumdis-2019-eular.7759
Candido J, Hagemann T (2013) Cancer-related inflammation. J Clin Immunol 33(Suppl 1):S79-84. https://doi.org/10.1007/s10875-012-9847-0
Scheau C, Badarau IA, Costache R et al (2019) The role of matrix metalloproteinases in the epithelial-mesenchymal transition of hepatocellular carcinoma. Anal Cell Pathol 2019:9423907. https://doi.org/10.1155/2019/9423907
Cioni C, Tassi M, Marotta G, Mugnaini C, Corelli F, Annunziata P (2019) A novel highly selective cannabinoid CB2 agonist reduces in vitro growth and tgf-beta release of human glial cell tumors. Cent Nerv Syst Agents Med Chem 19(3):206–214. https://doi.org/10.2174/1871524919666190923154351
Acknowledgements
None.
Funding
None declared.
Author information
Authors and Affiliations
Contributions
NO: Conceptualisation, drafting and editing of manuscript. OKY: Drafting of manuscript. AOA: Editing of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Osafo, N., Yeboah, O.K. & Antwi, A.O. Endocannabinoid system and its modulation of brain, gut, joint and skin inflammation. Mol Biol Rep 48, 3665–3680 (2021). https://doi.org/10.1007/s11033-021-06366-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06366-1