Abstract
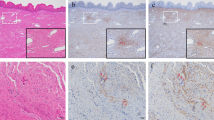
We evaluated the changes in the levels of TGF-β and SMAD gene and protein expression in the uterosacral ligament (USL) of patients with concomitant pelvic organ prolapse (POP) and urgency urinary incontinence (UUI) to illuminate the pathophysiology of UUI. The TGF‐β pathway is involved in collagen synthesis and degradation. The Transforming Growth Family-β (TGF‐β) superfamily has essential intracellular signaling components, such as newly identified SMAD family members. We evaluated the changes in the levels of TGF-β and SMAD gene and protein expression in the USL of patients with concomitant pelvic organ prolapse (POP) and UUI. This study included 10 patients who had been diagnosed with POP and UUI in the study group and 14 asymptomatic women without complaints of POP and UUI in the control group. Biopsy samples were collected from bilateral USL tissues during vaginal or abdominal hysterectomy. Total RNA was extracted from USL tissue and analyzed by qPCR. The protein expression levels were also analyzed with ELISA. In UUI patients, SMAD3 and TGF-ß1 gene expression levels significantly decreased compared to the control patients (p = 0.008 and p = 0.006, respectively). SMAD2 mRNA levels did not differ between the study and control groups (p = 0.139). No differences was found in the levels of SMAD2, SMAD3, and TGF-ß1 protein expression between the two groups. The reduction in the gene and protein expression levels of SMAD3 and TGF-ß1 in women with UUI and lax uterosacral ligaments may indicate a causal link.
Clinical trial registration: NCT04525105.

Similar content being viewed by others
References
Abrams P, Cardozo L, Fall M et al (2002) The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the international continence society. Neurourol Urodyn 21:167–178
Easley DC, Abramowitch SD, Moalli PA (2017) Female pelvic floor biomechanics: bridging the gap. Curr Opin Urol 27(3):262–267. https://doi.org/10.1097/MOU.0000000000000380
Liedl B, Markovsky O, Wagenlehner F, Gunnemann A (2012) The role of altered connective tissue in the causation of pelvic floor symptoms. Urinary Incontinence. Intech, Rijeka, pp 1–20
Petros PE, Ulmsten U (1993) An integral theory and its method, for the diagnosis and management of female urinary incontinence. Scand J Urol Nephrol 27:1–93
Petros PE, Ulmsten UI (1990) An integral theory of female urinary incontinence. Experimental and clinical considerations. Acta Obstet Gynecol Scand 69(S153):7–31
Alarab M, Kufaishi H, Lye S, Drutz H, Shynlova O (2014) Expression of extracellular matrix-remodeling proteins is altered in vaginal tissue of premenopausal women with severe pelvic organ prolapse. Reprod Sci 21:704–715
Ferrari MM, Rossi G, Biondi ML, Viganò P, Dell’utri C, Meschia M (2012) Type I collagen and matrix metalloproteinase 1, 3 and 9 gene polymorphisms in the predisposition to pelvic organ prolapse. Arch Gynecol Obstet 285:1581–1586
Ra HJ, Parks WC (2007) Control of matrix metalloproteinase catalytic activity. Matrix Biol 26:587–596
Tola EN, Koroglu N, Yıldırım GY, Koca HB (2018) The role of ADAMTS-2, collagen type-1, TIMP-3 and papilin levels of uterosacral and cardinal ligaments in the etiopathogenesis of pelvic organ prolapse among women without stres urinary incontinence. Eur J Obstet Gynecol Reprod Biol 231:158–163
Song Y, Hong X, Yu Y, Lin Y (2007) Changes of collagentype III and decorin in paraurethral connective tissue from women with stres urinary incontinence and prolapse. Int Urogynecol J Pelvic Floor Dysfunct 18:1459–1463
Ghosh AK, Yuan W, Mori Y, Varga J (2000) Smad-dependent stimulation of type I collagen gene expression in human skin fibroblasts by TGF-beta involves functional cooperation with p300/CBP transcriptional coactivators. Oncogene 19(31):3546–3555. https://doi.org/10.1038/sj.onc.1203693
Massagué J, Seoane J, Wotton D (2005) Smad transcription factors. Genes Dev 19(23):2783–2810. https://doi.org/10.1101/gad.1350705
Nakao A, Imamura T, Souchelnytskyi S et al (1997) TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J 16:5353–5362
Li GY, Cui WS, Zhou F et al (2012) Pathology of urethral fibromuscular system related to parturition-induced stres urinary incontinence and TGF-beta1/Smadpathway. Mol Cell Biochem 364:329–335
Wang H, Liu J, Zeng J, Zeng C, Zhou Y (2015) Expression of TβR-2, Smad3 and Smad7 in the vaginal anterior wall of postpartum rats with stres urinary incontinence. Arch Gynecol Obstet 291:869–876
Vetuschi A, Pompili S, Gallone A et al (2018) Immunolocalization of advanced glycation end products, mitogen activated protein kinases, and transforming growth factor-β/smads in pelvic organ prolapse. J Histochem Cytochem 66:673–686
Cam C, Sakalli M, Ay P, Cam M, Karateke A (2007) Validation of the short forms of the incontinence impact questionnaire (IIQ-7) and the urogenital distress inventory (UDI-6) in a Turkish population. Neurourol Urodyn 26:129–133
Bump RC, Mattiasson A, Bø K et al (1996) The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 175:10–17
Nandy S, Ranganathan S (2020) Urge ıncontinence. StatPearls Publishing, Treasure Island
Ludwig S, Becker I, Mallmann P, Jäger W (2019) Comparison of solifenacin and bilateral apical fixation in the treatment of mixed and urgency urinary ıncontinence in women: URGE 1 study, A randomized clinical trial. InVivo 33(6):1949–1957. https://doi.org/10.21873/invivo.11690
Ludwig S, Göktepe S, Mallmann P, Jäger W (2020) Evaluation of different “tensioning” of apical suspension in women undergoing surgery for prolapse and urinary incontinence. In Vivo 34(3):1371–1375. https://doi.org/10.21873/invivo.11916
Jackson SR, Avery NC, Tarlton JF, Eckford SD, Abrams P, Bailey AJ (1996) Changes in metabolism of collagen in genitourinary prolapse. Lancet 347:1658–1661
Klutke J, Ji Q, Campeau J et al (2008) Decreased endopelvic fascia elastin content in uterine prolapse. Acta Obstet Gynecol Scand 87:111–115
Chen B, Yeh J (2011) Alterations in connective tissue metabolism in stres incontinence and prolapse. J Urol 186:1768–1772
Gabriel B, Watermann D, Hancke K et al (2006) Increased expression of matrix metalloproteinase 2 in uterosacral ligaments is associated with pelvic organ prolapse. Int Urogynecol J 17:478–482
Strinic T, Vulic M, Tomic S, Capkun V, Stipic I, Alujevic I (2009) Matrix metalloproteinases-1, -2 expression in uterosacral ligaments from women with pelvic organ prolapse. Maturitas 64:132–135
Sun MJ, Cheng YS, Sun R, Cheng WL, Liu CS (2016) Changes in mitochondrial DNA copy number and extracellular matrix (ECM) proteins in the uterosacral ligaments of premenopausal women with pelvic organ prolapse. Taiwan J Obstet Gynecol 55:9–15
Heldin CH, Miyazono K, ten Dijke P (1997) TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 390(6659):465–471
Gong R, Xia Z (2019) Collagen changes in pelvic support tissues in women with pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol 234:185–189. https://doi.org/10.1016/j.ejogrb.2019.01.012
Urtasun R, Lopategi A, George J, Leung TM, Lu Y, Wang X et al (2012) Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin alpha(V) beta(3) engagement and PI3K/pAkt/NFkappaB signaling. Hepatology 55(2):594–608
Ge WS, Wang YJ, Wu JX, Fan JG, Chen YW, Zhu L (2014) Beta-catenin is over expressed in hepatic fibrosis and blockage of Wnt/beta-catenin signaling inhibits hepatic stellate cell activation. Mol Med Rep 9(6):2145–2151
Acknowledgements
This paper was supported by a grant from the Mugla Sitki Kocman University Research Projects Coordination Office (Project Grant Number 17/290).
Funding
This paper was supported by a grant from the Muğla Sıtkı Koçman University Research Projects Coordination Office (Project Grant Number 17/290).
Author information
Authors and Affiliations
Contributions
AAS: Project development, performing surgical procedure, manuscript writing. MNA: Project development, conceiving the study design and performing surgical procedure, data collection, manuscript writing. TE: carried out gene expression, Elisa and statistical analyses. SKC carried out gene expression, Elisa and statistical analyses. BK: contributed to writing and drafting the article, critical revision of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no confict of interest.
Ethical approval
Approval was obtained from the Institutional Review Board. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Informed consent
Written informed consent was obtained from all patients prior to their participation in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akin, M.N., Sivaslioglu, A.A., Edgunlu, T. et al. SMAD2, SMAD3 and TGF-β GENE expressions in women suffering from urge urinary incontinence and pelvic organ prolapse. Mol Biol Rep 48, 1401–1407 (2021). https://doi.org/10.1007/s11033-021-06220-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06220-4