Abstract
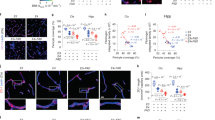
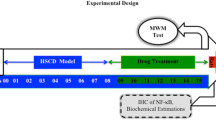
Advanced glycation end products (AGEs) are a group of modified proteins and/or lipids with damaging potential. AGEs-RAGE pathway plays a critical role to induce neurodegenerative encephalopathy. Statins can reduce the expression of AGEs-induced AGEs receptor (RAGE) in the aorta. It is not clear whether statins have potential benefits on AGEs-induced cognitive impairment. In this study, the effects of atorvastatin (ATV) on inflammation and oxidation stress in the cerebral cortex were investigated, and the underlying mechanisms were explored. Apolipoprotein E (ApoE)−/− male mice were divided into four groups: control, AGEs, AGEs + ALT711 (Alagebrium chloride) and AGEs + ATV. β-amyloid (Aβ) formation in the cerebral cortex was assessed through Congo red staining and the functional state of neurons was evaluated by Nissl’s staining. Immunostaining was performed to assess the accumulation of AGEs in the cerebral cortex. The expressions of mRNA and protein of RAGE, Nuclear factor kappa B (NF-κB) p65 and Nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) p47phox were detected by real-time polymerase chain reaction (PCR) and western blot. There were significant increases in AGEs deposit, Aβ formation, and the expressions of RAGE, NF-κB p65, and NADPH oxidase p47phox, and a decrease Nissl body in AGEs group compared with control group. ALT711 group recovered above change compared with AGEs group. Atorvastatin reduced Aβ formation and suppressed AGEs-induced expressions of NF-κB p65 and NADPH oxidase p47phox. Atorvastatin has little effects on AGEs deposit and RAGE expressions. Atorvastatin alleviates AGEs-induced neuronal impairment by alleviating inflammation and oxidative stress via inhibiting NADPH oxidase-NF-κB pathway.





Similar content being viewed by others
Data availability
The datasets used and analyzed during the current study are included in this published article.
Abbreviations
- AGEs:
-
Advanced glycation end products
- RAGE:
-
Receptor for AGEs
- NF-κB:
-
Nuclear factor kappa B
- ROS:
-
Reactive oxygen species
- NADPH oxidase:
-
Nicotinamide adenine dinucleotide phosphate-oxidase
- IL-1:
-
Interleukin-1
- IL-6:
-
Interleukin-6
- TNF-α:
-
Tumor necrosis factor-α
- Aβ:
-
β-Amyloid
- ApoE:
-
Apolipoprotein E
- ATV:
-
Atorvastatin
- BSA:
-
Bovine serum albumin
- SEM:
-
Standard error
- AD:
-
Alzheimer’s disease
References
Farhan SS, Hussain SA (2019) Advanced glycation end products (AGEs) and their soluble receptors (sRAGE) as early predictors of reno-vascular complications in patients with uncontrolled type 2 diabetes mellitus. Diabetes Metab Syndr 13(4):2457–2461. https://doi.org/10.1016/j.dsx.2019.06.019
Nowotny K, Jung T, Höhn A, Weber D, Grune T (2015) Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 5(1):194–222. https://doi.org/10.3390/biom5010194
Byun K, Yoo Y, Son M et al (2017) Advanced glycation end-products produced systemically and by macrophages: a common contributor to inflammation and degenerative diseases. Pharmacol Ther 177:44–55. https://doi.org/10.1016/j.pharmthera.2017.02.030
Shen C, Ma Y, Zeng Z et al (2017) RAGE-specific inhibitor FPS-ZM1 attenuates AGEs-induced neuroinflammation and oxidative stress in rat primary microglia. Neurochem Res 42(10):2902–2911. https://doi.org/10.1007/s11064-017-2321-x
Yeh CH, Sturgis L, Haidacher J et al (2001) Requirement for p38 and p44/p42 mitogen-activated protein kinases in RAGE-mediated nuclear factor-kappaB transcriptional activation and cytokine secretion. Diabetes 50(6):1495–1504. https://doi.org/10.2337/diabetes.50.6.1495
Singh R, Barden A, Mori T, Beilin L (2001) Advanced glycation end-products: a review. Diabetologia 44(2):129–146. https://doi.org/10.1007/s001250051591
Fan Q, Liao J, Kobayashi M et al (2004) Candesartan reduced advanced glycation end-products accumulation and diminished nitro-oxidative stress in type 2 diabetic KK/Ta mice. Nephrol Dialysis Transplant 19(12):3012–3020. https://doi.org/10.1093/ndt/gfh499
Yamagishi S, Ueda S, Matsui T, Nakamura K, Okuda S (2008) Role of advanced glycation end products (AGEs) and oxidative stress in diabetic retinopathy. Curr Pharm Des 14(10):962–968. https://doi.org/10.2174/138161208784139729
Srikanth V, Maczurek A, Phan T et al (2011) Advanced glycation endproducts and their receptor RAGE in alzheimer’s disease. Neurobiol Aging 32(5):763–777. https://doi.org/10.1016/j.neurobiolaging.2009.04.016
Bierhaus A, Humpert PM, Morcos M et al (2005) Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berlin, Germany) 83(11):876–886. https://doi.org/10.1007/s00109-005-0688-7
Bierhaus A, Nawroth PP (2009) Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia 52(11):2251–2263. https://doi.org/10.1007/s00125-009-1458-9
Bierhaus A, Schiekofer S, Schwaninger M et al (2001) Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes 50(12):2792–2808. https://doi.org/10.2337/diabetes.50.12.2792
Mattson MP, Camandola S (2001) NF-kappaB in neuronal plasticity and neurodegenerative disorders. J Clin Investig 107(3):247–254. https://doi.org/10.1172/JCI11916
Fang F, Yu Q, Arancio O et al (2018) RAGE mediates Aβ accumulation in a mouse model of alzheimer’s disease via modulation of β- and γ-secretase activity. Hum Mol Genet 27(6):1002–1014. https://doi.org/10.1093/hmg/ddy017
Wang L, Zhang X, Liu L, Yang R, Cui L, Li M (2010) Atorvastatin protects rat brains against permanent focal ischemia and downregulates HMGB1, HMGB1 receptors (RAGE and TLR4) NF-kappaB expression. Neurosci Lett 471(3):152–156. https://doi.org/10.1016/j.neulet.2010.01.030
Xu X, Gao W, Cheng S et al (2017) Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury. J Neuroinflammation 14(1):167. https://doi.org/10.1186/s12974-017-0934-2
Feng B, Xu L, Wang H et al (2011) Atorvastatin exerts its anti-atherosclerotic effects by targeting the receptor for advanced glycation end products. Biochem Biophys Acta 9:1130–1137. https://doi.org/10.1016/j.bbadis.2011.05.007
Xu L, Zang P, Feng B, Qian Q (2014) Atorvastatin inhibits the expression of RAGE induced by advanced glycation end products on aortas in healthy sprague-dawley rats. Diabetol Metab Syndr 6(1):102. https://doi.org/10.1186/1758-5996-6-102
Xu L, Wang YR, Li PC et al (2019) Atorvastatin blocks advanced glycation end products induced reduction in macrophage cholesterol efflux mediated with ATP-binding cassette transporters G 1. Circ J 83(9):1954–1964. https://doi.org/10.1253/circj.CJ-19-0153
Chen F, Ghosh A, Hu M et al (2018) RAGE-NF-κB-PPARγ signaling is involved in AGEs-induced upregulation of amyloid-β influx transport in an in vitro BBB model. Neurotox Res 33(2):284–299. https://doi.org/10.1007/s12640-017-9784-z
Schmidt AM, Weidman E, Lalla E et al (1996) Advanced glycation endproducts (AGEs) induce oxidant stress in the gingiva: a potential mechanism underlying accelerated periodontal disease associated with diabetes. J Periodontal Res 31(7):508–515. https://doi.org/10.1111/j.1600-0765.1996.tb01417.x
Li J, Wen PY, Li WW, Zhou J (2015) Upregulation effects of tanshinone IIA on the expressions of neuN, nissl body, and IκB and downregulation effects on the expressions of GFAP and NF-κB in the brain tissues of rat models of alzheimer’s disease. NeuroReport 26(13):758–766. https://doi.org/10.1097/WNR.0000000000000419
Chu H, Tang Y, Dong Q (2013) Protection of vascular endothelial growth factor to brain edema following intracerebral hemorrhage and its involved mechanisms: effect of aquaporin-4. PLoS ONE 8(6):e66051. https://doi.org/10.1371/journal.pone.0066051
Du LY, Chang LY, Ardiles AO et al (2015) Alzheimer’s disease-related protein expression in the retina of octodon degus. PLoS ONE 10(8):e0135499. https://doi.org/10.1371/journal.pone.0135499
Zhang KL, Lou DD, Guan ZZ (2015) Activation of the AGE/RAGE system in the brains of rats and in SH-SY5Y cells exposed to high level of fluoride might connect to oxidative stress. Neurotoxicol Teratol 48:49–55. https://doi.org/10.1016/j.ntt.2015.01.007
Wang P, Huang R, Lu S et al (2016) RAGE and AGEs in mild cognitive impairment of diabetic patients: a cross-sectional study. PLoS ONE 11(1):e0145521. https://doi.org/10.1371/journal.pone.0145521
Chuah YK, Basir R, Talib H et al (2013) Receptor for advanced glycation end products and its involvement in inflammatory diseases. Int J Inflammation 2013:403460. https://doi.org/10.1155/2013/403460
Chaney MO, Stine WB, Kokjohn TA et al (2005) RAGE and amyloid beta interactions: atomic force microscopy and molecular modeling. Biochem Biophys Acta 1741(1–2):199–205. https://doi.org/10.1016/j.bbadis.2005.03.014
Lubitz I, Ricny J, Atrakchi-Baranes D et al (2016) High dietary advanced glycation end products are associated with poorer spatial learning and accelerated Aβ deposition in an alzheimer mouse model. Aging Cell 15(2):309–316. https://doi.org/10.1111/acel.12436
Deane RJ (2012) Is RAGE still a therapeutic target for alzheimer’s disease? Future Med Chem 4(7):915–925. https://doi.org/10.4155/fmc.12.51
Li XH, Du LL, Cheng XS et al (2013) Glycation exacerbates the neuronal toxicity of β-amyloid. Cell death disease 4(6):e673. https://doi.org/10.1038/cddis.2013.180
Christen Y (2000) Oxidative stress and alzheimer disease. Am J Clin Nutr 71(2):621S-629S. https://doi.org/10.1093/ajcn/71.2.621s
Huang WJ, Zhang X, Chen WW (2016) Role of oxidative stress in alzheimer’s disease. Biomed Rep 4(5):519–522. https://doi.org/10.3892/br.2016.630
Liu C, Cui G, Zhu M et al (2014) Neuroinflammation in alzheimer’s disease: chemokines produced by astrocytes and chemokine receptors. Int J Clin Exp Pathol 7(12):8342–8355
Ramasamy R, Vannucci SJ, Yan SS et al (2005) Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 15(7):16R-28R. https://doi.org/10.1093/glycob/cwi053
Li J, Schmidt AM (1997) Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J Biol Chem 272(26):16498–16506. https://doi.org/10.1074/jbc.272.26.16498
Xu S, Zhang H, Yang X et al (2018) Inhibition of cathepsin L alleviates the microglia-mediated neuroinflammatory responses through caspase-8 and NF-κB pathways. Neurobiol Aging 62:159–167. https://doi.org/10.1016/j.neurobiolaging.2017.09.030
Yamamoto N, Fujii Y, Kasahara R et al (2016) Simvastatin and atorvastatin facilitates amyloid β-protein degradation in extracellular spaces by increasing neprilysin secretion from astrocytes through activation of MAPK/Erk1/2 pathways. Glia 64(6):952–962. https://doi.org/10.1002/glia.22974
Martins WC, dos Santos VV, dos Santos AA et al (2015) Atorvastatin prevents cognitive deficits induced by intracerebroventricular amyloid-β1-40 administration in mice: involvement of glutamatergic and antioxidant systems. Neurotox Res 28(1):32–42. https://doi.org/10.1007/s12640-015-9527-y
Wang S, Zhang X, Zhai L et al (2018) Atorvastatin attenuates cognitive deficits and neuroinflammation induced by Aβ1-42 involving modulation of TLR4/TRAF6/NF-κB pathway. J Mol Neurosci 64(3):363–373. https://doi.org/10.1007/s12031-018-1032-3
de Oliveira CV, Funck VR, Pereira LM et al (2013) Atorvastatin withdrawal elicits oxidative/nitrosative damage in the rat cerebral cortex. Pharmacol Res 71:1–8. https://doi.org/10.1016/j.phrs.2013.02.003
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Key Specialty Construction Project of Pudong Health and Family Planning Commission of Shanghai (PWZzk2017-12).
Author information
Authors and Affiliations
Contributions
ZL participated in the design of the study, carried out the immunohistochemistry, performed the statistical analysis and drafted the manuscript. PY carried out the animal experiment, RT-PCR and Western blot. BF conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
This study was conducted in accordance with the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health and with the approval by the Ethical Committee on Animal Research at Shanghai East Hospital of Tongji University (Shanghai, China).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Z., Yang, P. & Feng, B. Effect of atorvastatin on AGEs-induced injury of cerebral cortex via inhibiting NADPH oxidase -NF-κB pathway in ApoE−/− mice. Mol Biol Rep 47, 9479–9488 (2020). https://doi.org/10.1007/s11033-020-05998-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05998-z