Abstract
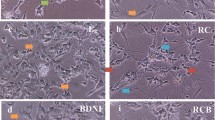
Numerous protocols to establish dopaminergic phenotype in SH-SY5Y cells have been reported. In most of these protocols there are variations in concentration of serum used. In this paper, we compared the effects of high (10%), low (3%) and descending (2.5%/1%) serum concentration in differentiation medium containing different proportion of retinoic acid (RA) and 12-O-Tetradecanoylphorbol-13-acetate (TPA) or RA-only on the undifferentiated SH-SY5Y cells with regards to cell morphology, biochemical and gene expression alterations. Cells differentiated in culture medium containing low and descending serum concentrations showed increased number of neurite projections and reduced proliferation rates when compared to undifferentiated cells. The SH-SY5Y cells differentiated in culture medium containing 3% RA and low serum or descending (2.5%/1% RA/TPA) were found to be more susceptible to 6-hydroxydopamine (6-OHDA) induced cytotoxicity. Cells differentiated with RA/TPA or RA differentiated showed increased production of the α-synuclein (SNCA) neuroprotein and dopamine neurotransmitter compared to undifferentiated cells, regardless serum concentrations used. There was no significant difference in the expression of tyrosine hydroxylase (TH) gene between undifferentiated and differentiated SH-SY5Y cells. However, the expression of dopamine receptor D2 (DRD2) gene was markedly increased (p<0.05) in differentiated cells with 3% serum and RA only when compared to undifferentiated cells. In conclusion, to terminally differentiate SH-SY5Y cells to be used as a cell-based model to study Parkinson’s disease (PD) to investigate molecular mechanisms and drug discovery, the optimal differentiation medium should contain 3% serum in RA-only






Similar content being viewed by others
Abbreviations
- RA:
-
retinoic acid
- TPA:
-
12-O-Tetradecanoylphorbol-13-acetate
- SH-SY5Y:
-
human neuroblastoma cell line
- 6-OHDA:
-
6-hydroxydopamine
- SNCA:
-
α-synuclein
- TH:
-
tyrosine hydroxylase
- DRD2:
-
dopamine receptor D2
- PD:
-
Parkinson’s disease
References
Dehay B (2015) Targeting α-synuclein for treating Parkinson’s disease: mechanistic and therapeutic consideration
Choi WS, Yoon SY, Oh TH et al (1999) Two distinct mechanisms are involved in 6-hydroxydopamine- and MPP+- induced dopaminergic neuronal cell death: Role of caspases, ROS, and JNK. J Neurosci Res 57:86–94. https://doi.org/10.1002/(SICI)1097-4547(19990701)57:1<86::AID-JNR9>3.0.CO;2-E
Whone AL, Watts RL, Stoessl AJ et al (2003) Slower progression of Parkinson’s disease with ropinirole versus levodopa: The REAL-PET study. Ann Neurol. https://doi.org/10.1002/ana.10609
Wooten GF (2003) Agonists vs levodopa in PD: The thrilla of whitha. Neurology
Cheung YT, Lau WKW, Yu MS et al (2009) Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 30:127–135. https://doi.org/10.1016/j.neuro.2008.11.001
Xicoy H, Wieringa B, Martens GJM (2017) The SH-SY5Y cell line in Parkinson’s disease research: a systematic review. Mol Neurodegener 12:1–11. https://doi.org/10.1186/s13024-017-0149-0
Kovalevich J, Langford D (2013) Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol Biol. https://doi.org/10.1007/978-1-62703-640-5_2
Biedler JL, Schachner M (1978) Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res 38:3751–3757
Xie HR, Sen HL, Li GY (2010) SH-SY5Y human neuroblastoma cell line: In vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin Med J (Engl) 123:1086–1092
Shipley MM, Mangold CA, Szpara ML (2016) Differentiation of the SH-SY5Y human neuroblastoma cell line. J Vis Exp 2016:1–11. https://doi.org/10.3791/53193
Hardwick LJA, Philpott A (2014) Nervous decision-making: To divide or differentiate. Trends Genet 30:254–261
Qiao J, Paul P, Lee S et al (2012) PI3K/AKT and ERK regulate retinoic acid-induced neuroblastoma cellular differentiation. Biochem Biophys Res Commun 424:421–426. https://doi.org/10.1016/j.bbrc.2012.06.125
Serdar B, Erkmen T, Ergür B et al (2020) Comparison of medium supplements in terms of the effects on the differentiation of SH-SY5Y human neuroblastoma cell line. Neurol Sci Neurophysiol 37:82. https://doi.org/10.4103/NSN.NSN_15_20
Goldie BJ, Barnett MM, Cairns MJ (2014) BDNF and the maturation of posttranscriptional regulatory networks in human SH-SY5Y neuroblast differentiation. Front Cell Neurosci 8:325. https://doi.org/10.3389/fncel.2014.00325
Feio-Azevedo R, Costa VM, Ferreira LM et al (2017) Toxicity of the amphetamine metabolites 4-hydroxyamphetamine and 4-hydroxynorephedrine in human dopaminergic differentiated SH-SY5Y cells. Toxicol Lett 269:65–76. https://doi.org/10.1016/j.toxlet.2017.01.012
Presgraves SP, Ahmed T, Borwege S, Joyce JN (2003) Terminally differentiated SH-SY5Y cells provide a model system for studying neuroprotective effects of dopamine agonists. Neurotox Res 5:579–598. https://doi.org/10.1007/BF03033178
Filograna R, Civiero L, Ferrari V et al (2015) Analysis of the catecholaminergic phenotype in human SH-SY5Y and BE(2)-M17 neuroblastoma cell lines upon differentiation. PLoS One 10:1–18. https://doi.org/10.1371/journal.pone.0136769
Costa-Mallen P, Costa LG, Smith-Weller T et al (2000) Genetic polymorphism of dopamine D2 receptors in Parkinson’s disease and interactions with cigarette smoking and MAO-B intron 13 polymorphism. J Neurol Neurosurg Psychiatry 69:535–537. https://doi.org/10.1136/jnnp.69.4.535
Ducray A, Wiedmer L, Herren F et al (2020) Quantitative characterization of phenotypical markers after differentiation of SH-SY5Y cells. CNS Neurol Disord - Drug Targets 19. https://doi.org/10.2174/1871527319666200708132716
Yang HN, Wang J, Sun JH et al (2016) A new method to effectively and rapidly generate neurons from SH-SY5Y cells. Neurosci Lett 610:43–47. https://doi.org/10.1016/j.neulet.2015.10.047
Khwanraj K, Phruksaniyom C, Madlah S, Dharmasaroja P (2015, 2015) Differential Expression of Tyrosine Hydroxylase Protein and Apoptosis-Related Genes in Differentiated and Undifferentiated SH-SY5Y Neuroblastoma Cells Treated with MPP+. Neurol Res Int. https://doi.org/10.1155/2015/734703
Lopes FM, Schröder R, da Júnior MLCF, et al (2010) Comparison between proliferative and neuron-like SH-SY5Y cells as an in vitro model for Parkinson disease studies. Brain Res 1337:85–94. https://doi.org/10.1016/j.brainres.2010.03.102
Fang CY, Wu CC, Fang CL et al (2017) Long-term growth comparison studies of FBS and FBS alternatives in six head and neck cell lines. PLoS One 12. https://doi.org/10.1371/journal.pone.0178960
Rashid M, Coombs KM (2019) Serum-reduced media impacts on cell viability and protein expression in human lung epithelial cells. J Cell Physiol 234:7718–7724. https://doi.org/10.1002/jcp.27890
Dwane S, Durack E, Kiely PA (2013) Optimising parameters for the differentiation of SH-SY5Y cells to study cell adhesion and cell migration. BMC Res Notes 6. https://doi.org/10.1186/1756-0500-6-366
Lo Surdo JL, Millis BA, Bauer SR (2013) Automated microscopy as a quantitative method to measure differences in adipogenic differentiation in preparations of human mesenchymal stromal cells. Cytotherapy. https://doi.org/10.1016/j.jcyt.2013.04.010
Berridge MV, Herst PM, Tan AS (2005) Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol Annu Rev
Taylor-Whiteley TR, Le Maitre CL, Duce JA et al (2019) Recapitulating Parkinson’s disease pathology in a three-dimensional human neural cell culture model. DMM Dis Model Mech 12. https://doi.org/10.1242/dmm.038042
Wang T, Ye X, Bian W et al (2020) Allopregnanolone Modulates GABAAR-Dependent CaMKIIδ3 and BDNF to Protect SH-SY5Y Cells Against 6-OHDA-Induced Damage. Front Cell Neurosci 13:569. https://doi.org/10.3389/fncel.2019.00569
Ramalingam M, Huh YJ, Lee Y Il (2019) The Impairments of α-Synuclein and Mechanistic Target of Rapamycin in Rotenone-Induced SH-SY5Y Cells and Mice Model of Parkinson’s Disease. Front Neurosci 13:1028. https://doi.org/10.3389/fnins.2019.01028
Shimohama S, Sawada H, Kitamura Y, Taniguchi T (2003) Disease model: Parkinson’s disease. Trends Mol Med 9:360–365. https://doi.org/10.1016/S1471-4914(03)00117-5
Kovalevich J, Langford D (2013) Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol Biol 1078:9–21. https://doi.org/10.1007/978-1-62703-640-5_2
Voigt A, Zintl F (2003) Effects of retinoic acid on proliferation, apoptosis, cytotoxicity, migration, and invasion of neuroblastoma cells. Med Pediatr Oncol 40:205–213. https://doi.org/10.1002/mpo.10250
Forster JI, Köglsberger S, Trefois C et al (2016) Characterization of differentiated SH-SY5Y as neuronal screening model reveals increased oxidative vulnerability. J Biomol Screen 21:496–509. https://doi.org/10.1177/1087057115625190
Qiao J, Paul P, Lee S et al (2012) PI3K/AKT and ERK regulate retinoic acid-induced neuroblastoma cellular differentiation. Biochem Biophys Res Commun. https://doi.org/10.1016/j.bbrc.2012.06.125
Pan J, Kao YL, Joshi S et al (2005) Activation of Rac1 by phosphatidylinositol 3-kinase in vivo: Role in activation of mitogen-activated protein kinase (MAPK) pathways and retinoic acid-induced neuronal differentiation of SH-SY5Y cells. J Neurochem. https://doi.org/10.1111/j.1471-4159.2005.03106.x
Wiese C, Rolletschek A, Kania G et al (2004) Nestin expression - A property of multi-lineage progenitor cells? Cell Mol Life Sci 61:2510–2522
Hendrickson ML, Rao AJ, Demerdash ONA, Kalil RE (2011) Expression of nestin by neural cells in the adult rat and human brain. PLoS One 6:e18535. https://doi.org/10.1371/journal.pone.0018535
Lendahl U, Zimmerman LB, McKay RDG (1990) CNS stem cells express a new class of intermediate filament protein. Cell 60:585–595. https://doi.org/10.1016/0092-8674(90)90662-X
Hu W, Lu H, Wang S et al (2016) Suppression of Nestin reveals a critical role for p38-EGFR pathway in neural progenitor cell proliferation. Oncotarget 7:87052–87063. https://doi.org/10.18632/oncotarget.13498
Bernal A, Arranz L (2018) Nestin-expressing progenitor cells: function, identity and therapeutic implications. Cell Mol Life Sci 75:2177–2195
Kunzler A, Zeidán-Chuliá F, Gasparotto J et al (2017) Changes in cell cycle and up-regulation of neuronal markers during SH-SY5Y neurodifferentiation by retinoic acid are mediated by reactive species production and oxidative stress. Mol Neurobiol 54. https://doi.org/10.1007/s12035-016-0189-4
Salama M, Arias Carrion O (2011) Natural toxins implicated in the development of Parkinson’s disease. Ther Adv Neurol Disord. https://doi.org/10.1177/1756285611413004
Duty S, Jenner P (2011) Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol 164:1357–1391. https://doi.org/10.1111/j.1476-5381.2011.01426.x
Funakohi-Tago M, Sakata T, Fujiwara S et al (2018) Hydroxytyrosol butyrate inhibits 6-OHDA-induced apoptosis through activation of the Nrf2/HO-1 axis in SH-SY5Y cells. Eur J Pharmacol 834:246–256. https://doi.org/10.1016/j.ejphar.2018.07.043
Magalingam KB, Radhakrishnan A, Ramdas P, Haleagrahara N (2014) Quercetin glycosides induced neuroprotection by changes in the gene expression in a cellular model of Parkinson’s disease. J Mol Neurosci 55:609–617. https://doi.org/10.1007/s12031-014-0400-x
Bindu J, Das A, Sakthivel KM (2020) Anthraquinone from Edible Fungi Pleurotus ostreatus Protects Human SH-SY5Y Neuroblastoma Cells Against 6-Hydroxydopamine-Induced Cell Death—Preclinical Validation of Gene Knockout Possibilities of PARK7, PINK1, and SNCA1 Using CRISPR SpCas9. Appl Biochem Biotechnol 191:555–566. https://doi.org/10.1007/s12010-019-03188-7
Lopes FM, da Motta LL, De Bastiani MA et al (2017) RA differentiation enhances dopaminergic features, changes redox parameters, and increases dopamine transporter dependency in 6-Hydroxydopamine-induced neurotoxicity in SH-SY5Y Cells. Neurotox Res. https://doi.org/10.1007/s12640-016-9699-0
Silva J, Alves C, Pinteus S et al (2018) Neuroprotective effects of seaweeds against 6-hydroxidopamine-induced cell death on an in vitro human neuroblastoma model. BMC Complement Altern Med. https://doi.org/10.1186/s12906-018-2103-2
Kragh CL, Romero-Ramos M, Halliday G, Jensen PH (2014) Alpha synuclein in parkinson’s disease. Handb Neurotox 2:691–726. https://doi.org/10.1007/978-1-4614-5836-4_14
Bridi JC, Hirth F (2018) Mechanisms of α-Synuclein induced synaptopathy in parkinson’s disease. Front Neurosci 12:80
Burré J (2015) The synaptic function of α-synuclein. J Parkinsons Dis 5:699–713
Kim WS, Kagedal K, Halliday GM (2014) Alpha-synuclein biology in Lewy body diseases. Alzheimer’s Res Ther 6:1–9. https://doi.org/10.1186/s13195-014-0073-2
Sanderson JB, De S, Jiang H et al (2020) Analysis of α-synuclein species enriched from cerebral cortex of humans with sporadic dementia with Lewy bodies. Brain Commun:2. https://doi.org/10.1093/braincomms/fcaa010
Xu L, Pu J (2016) Alpha-synuclein in Parkinson’s disease: from pathogenetic dysfunction to potential clinical application. Parkinsons. Dis.
Jang A, Lee HJ, Suk JE et al (2010) Non-classical exocytosis of α-synuclein is sensitive to folding states and promoted under stress conditions. J Neurochem. https://doi.org/10.1111/j.1471-4159.2010.06695.x
Angot E, Steiner JA, Lema Tomé CM et al (2012) Alpha-synuclein cell-to-cell transfer and seeding in grafted dopaminergic neurons In Vivo. PLoS One 7:e39465. https://doi.org/10.1371/journal.pone.0039465
Alvarez-Erviti L, Seow Y, Schapira AH et al (2011) Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis 42:360–367. https://doi.org/10.1016/j.nbd.2011.01.029
Daubner SC, Le T, Wang S (2011) Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys
Luo SX, Huang EJ (2016) Dopaminergic neurons and brain reward pathways: From neurogenesis to circuit assembly. Am J Pathol 186:478–488
Juárez Olguín H, Calderón Guzmán D, Hernández García E, Barragán Mejía G (2016) The role of dopamine and its dysfunction as a consequence of oxidative stress. Oxid Med Cell, Longev
An JH, Oh BK, Choi JW (2013) Detection of tyrosine hydroxylase in dopaminergic neuron cell using gold nanoparticles-based barcode DNA. J Biomed Nanotechnol. https://doi.org/10.1166/jbn.2013.1525
German CL, Baladi MG, McFadden LM et al (2015) Regulation of the dopamine and vesicular monoamine transporters: pharmacological targets and implications for disease. Pharmacol Rev 67:1005–1024
Bhatia A, Saadabadi A (2019) Biochemistry. StatPearls Publishing, Dopamine Receptors
Alexander GE (2004) Biology of Parkinson’s disease: Pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin Neurosci 6:259–280
Lopes FM, da Motta LL, De Bastiani MA et al (2017) RA differentiation enhances dopaminergic features, changes redox parameters, and increases dopamine transporter dependency in 6-Hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. Neurotox Res 31. https://doi.org/10.1007/s12640-016-9699-0
Korecka JA, van Kesteren RE, Blaas E et al (2013) Phenotypic characterization of retinoic acid differentiated SH-SY5Y cells by transcriptional profiling. PLoS One. https://doi.org/10.1371/journal.pone.0063862
Nagatsu T, Nagatsu I (2016) Tyrosine hydroxylase (TH), its cofactor tetrahydrobiopterin (BH4), other catecholamine-related enzymes, and their human genes in relation to the drug and gene therapies of Parkinson’s disease (PD): historical overview and future prospects. J Neural Transm 123:1255–1278
Masato A, Plotegher N, Boassa D, Bubacco L (2019) Impaired dopamine metabolism in Parkinson’s disease pathogenesis. Mol Neurodegener 14:1–21
Khwanraj K, Phruksaniyom C, Madlah S, Dharmasaroja P (2015) Differential expression of tyrosine hydroxylase protein and apoptosis-related genes in differentiated and undifferentiated SH-SY5Y neuroblastoma cells treated with MPP+. Neurol Res Int. https://doi.org/10.1155/2015/734703
Glanemann C, Loos A, Gorret N et al (2003) Disparity between changes in mRNA abundance and enzyme activity in Corynebacterium glutamicum: Implications for DNA microarray analysis. Appl Microbiol Biotechnol. https://doi.org/10.1007/s00253-002-1191-5
CARLSSON A, FALCK B, HILLARP NA (1962) Cellular localization of brain monoamines. Acta Physiol Scand Suppl
DAI D, WANG Y, WANG L et al (2014) Polymorphisms of DRD2 and DRD3 genes and Parkinson’s disease: A meta-analysis. Biomed Reports 2:275–281. https://doi.org/10.3892/br.2014.220
Oliveri RL, Annesi G, Zappia M et al (2000) The dopamine D2 receptor gene is a susceptibility locus for Parkinson’s disease. Mov Disord 15:120–126. https://doi.org/10.1002/1531-8257(200001)15:1<120::aid-mds1019>3.0.co;2-s
Hauge XY, Grandy DK, Eubanks JH et al (1991) Detection and characterization of additional DNA polymorphisms in the dopamine D2 receptor gene. Genomics 10:527–530. https://doi.org/10.1016/0888-7543(91)90431-D
Shioda N (2017) Dopamine D2L receptor-interacting proteins regulate dopaminergic signaling. J Pharmacol Sci 135:51–54. https://doi.org/10.1016/j.jphs.2017.10.002
Baik JH, Picetti R, Saiardi A et al (1995) Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature
Tinsley RB, Bye CR, Parish CL et al (2009) Dopamine D2 receptor knockout mice develop features of Parkinson disease. Ann Neurol. https://doi.org/10.1002/ana.21716
Double KL, Crocker AD (1995) Dopamine receptors in the substantia nigra are involved in the regulation of muscle tone. Proc Natl Acad Sci U S A 92:1669–1673. https://doi.org/10.1073/pnas.92.5.1669
Acknowledgements
The authors are very grateful to Dr Premdass Ramdas for his helpful guidance and technical assistance in the PCR assays.
Funding
This study was supported by International Medical University, Kuala Lumpur research grant (Grant: IMU R 194 2016).
Author information
Authors and Affiliations
Contributions
All authors whose names appear on the submission contributed to the study conception, design of the work, analysis and interpretation of data. All authors read and approved the version to be published.
Corresponding author
Ethics declarations
Conflict of Interest
Authors Kasthuri Bai Magalingam, Ammu Kutty Radhakrishnan, Sushela Devi Somanath, Shadab Md, and Nagaraja Haleagrahara declare that we have no conflict of interest.
Ethical approval
This study was approved by the International Medical University, Institutional Research Ethics Committee. This article does not contain any studies with human participants or animals performed by any of the authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Magalingam, K.B., Radhakrishnan, A.K., Somanath, S.D. et al. Influence of serum concentration in retinoic acid and phorbol ester induced differentiation of SH-SY5Y human neuroblastoma cell line. Mol Biol Rep 47, 8775–8788 (2020). https://doi.org/10.1007/s11033-020-05925-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05925-2