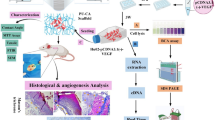
Abstract
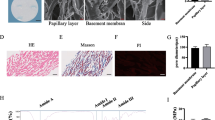
Cell-based wound therapy is faced with some limiting factors that decrease the therapeutic efficacy of transplanted cells. In this study, we aimed to genetically modify fibroblast cells with anti-apoptotic Survivin gene (Birc5) before cell transplantation. In vitro, pIRES2-eGFP-Survivin plasmid was transfected into the fibroblast cells and the growth curve was evaluated for transfected and normal cells performing MTT assay. In vivo, two 6-diameter cutaneous wounds were created at mice dorsal skin. Fibrin clot was used as a delivery vehicle to transfer cells into the wound bed. The effects of four treatment groups including (a) Cell-SVV-Clot (b) Cell-GFP-Clot, (c) Normal cell-Clot and, (d) Clot alone were evaluated. After 1,2,3,7 and 14 days post-transplantation, the wounds were photographed for evaluating the wound closure rate and wound samples were obtained. Angiogenesis and formation of granulated tissue were assessed via H&E staining for wound samples. The expression levels of Survivin, VEGF, and bFGF genes were also determined using qRT-PCR. The MTT assay showed similar proliferation potential of transfected cells with normal cells verifying that Survivin had no detrimental effect. Compared to the Normal cell-Clot group, the Survivin overexpression was seen for 3 days in the Cell-SVV-Clot group verifying the cell survival during the early stage of wound healing. The Survivin further upregulated VEGF and bFGF expressions resulting in more angiogenesis and formation of granulated tissue by day 3 and 14. The treated wounds with Cell-SVV-Clot were regenerated with a higher wound closure rate by day 7 compared to Normal cell-Clot and Clot groups. Survivin enhanced wound healing through induction of VEGF and bFGF at particular times post-wounding that led to a more structured-epidermis with higher angiogenesis and granulation tissue formation rate.









Similar content being viewed by others
References
Mori Y, Nakagami G, Kitamura A et al (2019) Effectiveness of biofilm-based wound care system on wound healing in chronic wounds. Wound Repair Regen 27(5):540–547
Kim WJ, Mohan RR, Mohan RR, Wilson SE (1999) Effect of PDGF, IL-1alpha, and BMP2/4 on corneal fibroblast chemotaxis: expression of the platelet-derived growth factor system in the cornea. Invest Ophthalmol Vis Sci 40(7):1364–1372
Li B, Wang JHC (2011) Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J Tissue Viability 20(4):108–120
Yates CC, Rodrigues M, Nuschke A et al (2017) Multipotent stromal cells / mesenchymal stem cells and fibroblasts combine to minimize skin hypertrophic scarring. Stem Cell Res Ther 8(1):193
Xue X, Liu Y, Zhang J, Liu T, Yang Z, Wang H (2015) Bcl-xL genetic modification enhanced the therapeutic efficacy of mesenchymal stem cell transplantation in the treatment of heart infarction. Stem Cells Int 2015:176409
McKenzie JA, Grossman D (2012) Role of the apoptotic and mitotic regulator survivin in melanoma. Anticancer Res 32(2):397–404
Jha K, Shukla M, Pandey M (2012) Survivin expression and targeting in breast cancer. Surg Oncol 21(2):125–131
Shojaei F, Yazdani-Nafchi F, Banitalebi-Dehkordi M, Chehelgerdi M, Khorramian-Ghahfarokhi M (2018) Trace of survivin in cancer. Eur J Cancer Prev 28(4):365–372
Assadiasl S, Mousavi MJ, Amirzargar A (2018) Antiapoptotic molecule survivin in transplantation: helpful or harmful? J Transplant 2018:1–6
Przybylski M (2009) A review of the current research on the role of bFGF and VEGF in angiogenesis. J Wound Care 18(12):516–519
Miller ED, Song F, Smith JD et al (2018) Plasma-based biomaterials for the treatment of cutaneous radiation injury. Wound Repair Regen 27(2):139–149
Clover AJP, Kumar AHS, Isakson M et al (2015) Allogeneic mesenchymal stem cells, but not culture modified monocytes, improve burn wound healing. Burns 41(3):548–557
Chang A, Chau V, Landas J (2017) Preparation of calcium competent Escherichia coli and heat-shock transformation. JEMI methods 1(June):22–25
Eyrich D, Brandl F, Appel B et al (2007) Long-term stable fibrin gels for cartilage engineering. Biomaterials 28(1):55–65
Buceviˇ J, Lukinaviˇ G (2018) The use of hoechst dyes for dna staining and beyond. Chemosensors. https://doi.org/10.3390/chemosensors6020018
Clouthier S, Luther T, Wicha M. Ketamine/Xylazine Containing Anesthesia for Mouse Surgery Preparation. Available from: https://www.med.umich.edu/wicha-lab/SOP/SOP 6.3- Ketamine Xylazine Anesthesia 4–2–2015.pdf.
Fischer AH, Jacobson KA, Rose J, Zeller R. (2008) Hematoxylin and Eosin Staining of Tissue and Cell Sections. Cold Spring Harb. Protoc. 2008(5), pdb.prot4986.
Chen J, Chen J-K, Conway EM, Harris RC (2013) Survivin mediates renal proximal tubule recovery from AKI. J Am Soc Nephrol 24(12):2023–2033
Kindt N, Menzebach A, Van de Wouwer M, Betz I, De Vriese A, Conway EM (2007) Protective role of the inhibitor of apoptosis protein, survivin, in toxin-induced acute renal failure. FASEB J 22(2):510–521
Velander P, Theopold C, Bleiziffer O et al (2009) cell suspensions of autologous keratinocytes or autologous fibroblasts accelerate the healing of full thickness skin wounds in a diabetic porcine wound healing model. J Surg Res 157(1):14–20
Kouhbananinejad SM, Derakhshani A, Vahidi R et al (2019) A fibrinous and allogeneic fibroblast-enriched membrane as a biocompatible material can improve diabetic wound healing. Biomater Sci 7(5):1949–1961
Cengiz C, Soyocak A, Karabag Y (2011) The effects of combined application of autogenous fibroblast cell culture and full-tissue skin graft (FTSG) on wound healing and contraction in full-thickness tissue defects e Aydan Ko. Burns 8:2–8
Şakrak T, Köse AA, Kivanç Ö et al (2012) The effects of combined application of autogenous fibroblast cell culture and full-tissue skin graft (FTSG) on wound healing and contraction in full-thickness tissue defects. Burns 38(2):225–231
Havasi A, Borkan SC (2011) Apoptosis and acute kidney injury. Kidney Int 80(1):29–40
Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI (2005) Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol 46(7):1339–1350
Fan L, Lin C, Zhuo S et al (2009) Transplantation with survivin-engineered mesenchymal stem cells results in better prognosis in a rat model of myocardial infarction. Eur J Heart Fail 11(11):1023–1030
Liu N, Zhang Y, Fan L et al (2011) Effects of transplantation with bone marrow-derived mesenchymal stem cells modified by Survivin on experimental stroke in rats. J. Transl. Med. https://doi.org/10.1186/1479-5876-9-105
Steinberg JP, Hong SJ, Matthew R, Galiano RD, Mustoe TA (2012) Equivalent effects of topically-delivered adipose-derived stem cells and dermal fibroblasts in the ischemic rabbit ear model for chronic wounds. Aesthet Surg J 32(4):504–519
Losi P, Briganti E, Errico C et al (2013) Acta biomaterialia fibrin-based scaffold incorporating VEGF- and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater 9(8):7814–7821
Lohmeyer JA, Liu F, Krüger S, Lindenmaier W, Siemers F, MacHens HG (2011) Use of gene-modified keratinocytes and fibroblasts to enhance regeneration in a full skin defect. Langenbeck’s Archives of Surgery 396:543–550
Eggers C, Mu J (2015) VEGF transfer based on gene- modified fibroblasts using a hypoxia-induced vector to modulate neoangiogenesis in ischaemic regions of myocutaneous transplants. Int J Oral Maxillofac Surg. https://doi.org/10.1016/j.ijom.2014.06.018
Chang J, Most D, Bresnick S et al (1999) Proliferative hemangiomas: analysis of cytokine gene expression and angiogenesis. Plast Reconstr Surg 103(1):1–10
Birkenhauer E, Neethirajan S (2015) A double-edged sword: the role of VEGF in wound repair and chemoattraction of opportunist pathogens. Int J Mol Sci 16(4):7159–7172
Hirsch T, von Peter S, Dubin G et al (2006) Adenoviral gene delivery to primary human cutaneous cells and burn wounds. Mol Med 12(9–10):199–207
Makino T, Jinnin M, Muchemwa FC et al (2010) Basic fibroblast growth factor stimulates the proliferation of human dermal fibroblasts via the ERK 1⁄2 and JNK pathways. Br J Dermatol 162(4):717–723
Kanazawa S, Fujiwara T, Matsuzaki S, Shingaki K, Taniguchi M (2010) bFGF regulates PI3-kinase-rac1-JNK pathway and promotes fibroblast migration in wound healing. PLoS ONE 5(8):1–12
Okumura M, Okuda T, Okamoto T, Nakamura T, Yajima M (1996) Enhanced angiogenesis and granulation tissue formation by basic fibroblast growth factor in healing-impaired animals. Arzneimittelforschung 46(10):1021–1026
Okumuram OT, Nakamura T, Yajima M (2011) Acceleration of wound healing in diabetic mice by basic fibroblast growth factor. Biol Pharm Bull 19(4):530–535
Akasaka Y, Ono I, Yamashita T, Jimbow K, Ishii T (2004) Basic fibroblast growth factor promotes apoptosis and suppresses granulation tissue formation in acute incisional wounds. J Pathol 203(2):710–720
Acknowledgements
The authors thank the Cellular and Molecular Research Center of Shahrekord University of Medical Sciences for their supports.
Funding
This research was funded by Shahrekord University of Medical Sciences (Grant Number:1396-08-74-516).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Authors declare that they have no conflict of interest.
Research involving animals
All animal intervention in this study was carried out according to the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86-23, Revised 1985). The ethics code was also received from Ethics Committee of Shahrekord University of Medical Sciences for animal experiments in this research. (Ethics code: IR.SKUMS.RE.1396.203).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shojaei-Ghahrizjani, F., Rahmati, S., Mirzaei, S.A. et al. Does survivin overexpression enhance the efficiency of fibroblast cell-based wound therapy?. Mol Biol Rep 47, 5851–5864 (2020). https://doi.org/10.1007/s11033-020-05656-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05656-4