Abstract
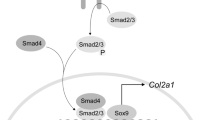
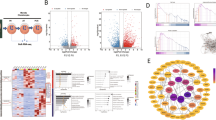
Chondrocytes are the sole cell type present within cartilage, and play pivotal roles in controlling the formation and composition of health cartilage. Chondrocytes maintain cartilage homeostasis through proliferating, differentiating and synthesizing different types of extracellular matrices. Thus, the coordinated proliferation and differentiation of chondrocytes are essential for cartilage growth, repair and the conversion from cartilage to bone during the processes of bone formation and fracture healing. Runx3, a transcription factor that belongs to the Runx family, is significantly upregulated at the onset of cartilage mineralization and regulates both early and late markers of chondrocyte maturation. Therefore, Runx3 may serve as an accelerator of chondrocyte differentiation and maturation. However, the underlying molecular mechanism of Runx3 in regulating chondrocyte proliferation and differentiation remains largely to be elucidated. In the present study, we used state-of-the-art RNA-seq technology combined with validation methods to investigate the effect of Runx3 overexpression or silencing on primary chondrocyte proliferation and differentiation, and demonstrated that Runx3 overexpression possibly inhibited chondrocyte proliferation but accelerated differentiation, whereas Runx3 silencing possibly promoted chondrocyte proliferation but suppressed differentiation. Furthermore, Runx3 overexpression possibly decreased the expression levels of Sox9 and its downstream genes via Sox9 cartilage-specific enhancers, and vice versa for Runx3 silencing.








Similar content being viewed by others
References
Goldring MB (2012) Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther Adv Musculoskelet Dis 4:269–285
Maldonado M, Nam J (2013) The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed Res Int 2013:284873
Wuelling M, Vortkamp A (2011) Chondrocyte proliferation and differentiation. Endocr Dev 21:1–11
Tsang KY, Cheah KS (2019) The extended chondrocyte lineage: implications for skeletal homeostasis and disorders. Curr Opin Cell Biol 61:132–140
Goldring MB, Marcu KB (2009) Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther 11:224
Akkiraju H, Nohe A (2015) Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. J Dev Biol 3:177–192
Hu DP, Ferro F, Yang F, Taylor AJ, Chang W, Miclau T, Marcucio RS, Bahney CS (2017) Cartilage to bone transformation during fracture healing is coordinated by the invading vasculature and induction of the core pluripotency genes. Development 144:221–234
Hinton RJ, Jing Y, Jing J, Feng JQ (2017) Roles of chondrocytes in endochondral bone formation and fracture repair. J Dent Res 96:23–30
Aghajanian P, Mohan S (2018) The art of building bone: emerging role of chondrocyte-to-osteoblast transdifferentiation in endochondral ossification. Bone Res 6:19
Wuelling M, Vortkamp A (2010) Transcriptional networks controlling chondrocyte proliferation and differentiation during endochondral ossification. Pediatr Nephrol 25:625–631
Yoshida CA, Yamamoto H, Fujita T, Furuichi T, Ito K, Inoue K, Yamana K, Zanma A, Takada K, Ito Y, Komori T (2004) Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev 18:952–963
Soung do Y, Dong Y, Wang Y, Zuscik MJ, Schwarz EM, O'Keefe RJ, Drissi H (2007) Runx3/AML2/Cbfa3 regulates early and late chondrocyte differentiation. J Bone Miner Res 22:1260–1270
Stark R, Grzelak M, Hadfield J (2019) RNA sequencing: the teenage years. Nat Rev Genet 20:631–656
Spies D, Renz PF, Beyer TA, Ciaudo C (2019) Comparative analysis of differential gene expression tools for RNA sequencing time course data. Brief Bioinform 20:288–298
Yao B, Wang Q, Liu CF, Bhattaram P, Li W, Mead TJ, Crish JF, Lefebvre V (2015) The SOX9 upstream region prone to chromosomal aberrations causing campomelic dysplasia contains multiple cartilage enhancers. Nucleic Acids Res 43:5394–5408
Yao B, Zhang M, Leng X, Liu M, Liu Y, Hu Y, Zhao D, Zhao Y (2018) Antler extracts stimulate chondrocyte proliferation and possess potent anti-oxidative, anti-inflammatory, and immune-modulatory properties. In Vitro Cell Dev Biol Anim 54:439–448
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C (T) method. Nat Protoc 3:1101–1108
Kim D, Langmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12:357–360
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28:511–515
Wang L, Feng Z, Wang X, Wang X, Zhang X (2010) DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26:136–138
Li J, Dong S (2016) The signaling pathways involved in chondrocyte differentiation and hypertrophic differentiation. Stem Cells Int 2016:2470351
Zuscik MJ, Hilton MJ, Zhang X, Chen D, O'Keefe RJ (2008) Regulation of chondrogenesis and chondrocyte differentiation by stress. J Clin Invest 118:429–438
Yong H, Zhao W, Zhou X, Liu Z, Tang Q, Shi H, Cheng R, Zhang X, Qiu Z, Zhu J, Feng Z (2019) RNA-Binding Motif 4 (RBM4) Suppresses Tumor Growth and Metastasis in Human Gastric Cancer. Med Sci Monit 25:4025–4034
Wu XN, Shi TT, He YH, Wang FF, Sang R, Ding JC, Zhang WJ, Shu XY, Shen HF, Yi J, Gao X, Liu W (2017) Methylation of transcription factor YY2 regulates its transcriptional activity and cell proliferation. Cell Discov 3:17035
Steinbusch MM, Caron MM, Eckmann F, Lausch E, van Rhijn LW, Zabel B, Welting TJ (2015) Viperin; a novel chondrogenic regulator. Osteoarthr Cartilage 23:A148–A149
Al-Hilal TA, Chung SW, Choi JU, Alam F, Park J, Kim SW, Kim SY, Ahsan F, Kim IS, Byun Y (2016) Targeting prion-like protein doppel selectively suppresses tumor angiogenesis. J Clin Invest 126:1251–1266
Yee NS (2015) Roles of TRPM8 ion channels in cancer: proliferation, survival, and invasion. Cancers (Basel) 7:2134–2146
Sukumaran P, Löf C, Pulli I, Kemppainen K, Viitanen T, Törnquist K (2013) Significance of the transient receptor potential canonical 2 (TRPC2) channel in the regulation of rat thyroid FRTL-5 cell proliferation, migration, adhesion and invasion. Mol Cell Endocrinol 374:10–21
Bloch DB, Nakajima A, Gulick T, Chiche JD, Orth D, de La Monte SM, Bloch KD (2000) Sp110 localizes to the PML-Sp100 nuclear body and may function as a nuclear hormone receptor transcriptional coactivator. Mol Cell Biol 20:6138–6146
Posey KL, Hecht JT (2008) The role of cartilage oligomeric matrix protein (COMP) in skeletal disease. Curr Drug Targets 9:869–877
Zhang W, Cheng P, Hu W, Yin W, Guo F, Chen A, Huang H (2018) Downregulated microRNA-340-5p promotes proliferation and inhibits apoptosis of chondrocytes in osteoarthritis mice through inhibiting the extracellular signal-regulated kinase signaling pathway by negatively targeting the FMOD gene. J Cell Physiol 234:927–939
Chen Y, Cossman J, Jayasuriya CT, Li X, Guan Y, Fonseca V, Yang K, Charbonneau C, Yu H, Kanbe K, Ma P, Darling E, Chen Q (2016) Deficient Mechanical activation of anabolic transcripts and post-traumatic cartilage degeneration in matrilin-1 knockout mice. PLoS ONE 11:e0156676
Komori T (2018) Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem Cell Biol 149:313–323
Gasparini G, De Gori M, Paonessa F, Chiefari E, Brunetti A, Galasso O (2012) Functional relationship between high mobility group A1 (HMGA1) protein and insulin-like growth factor-binding protein 3 (IGFBP-3) in human chondrocytes. Arthritis Res Ther 14:R207
Fransès RE, McWilliams DF, Mapp PI, Walsh DA (2010) Osteochondral angiogenesis and increased protease inhibitor expression in OA. Osteoarthr Cartilage 18:563–571
Guévremont M, Martel-Pelletier J, Boileau C, Liu FT, Richard M, Fernandes JC, Pelletier JP, Reboul P (2004) Galectin-3 surface expression on human adult chondrocytes: a potential substrate for collagenase-3. Ann Rheum Dis 63:636–643
White MJ, Roife D, Gomer RH (2015) Galectin-3 binding protein secreted by breast cancer cells inhibits monocyte-derived fibrocyte differentiation. J Immunol 195:1858–1867
Recklies AD, Ling H, White C, Bernier SM (2005) Inflammatory cytokines induce production of CHI3L1 by articular chondrocytes. J Biol Chem 280:41213–41221
Jiang Y, Tuan RS (2019) Role of NGF-TrkA signaling in calcification of articular chondrocytes. FASEB J 33:10231–10239
Iannone F, De Bari C, Dell'Accio F, Covelli M, Patella V, Lo Bianco G, Lapadula G (2002) Increased expression of nerve growth factor (NGF) and high affinity NGF receptor (p140 TrkA) in human osteoarthritic chondrocytes. Rheumatology (Oxford) 41:1413–1418
Rose BJ, Kooyman DL (2016) A tale of two joints: the role of matrix metalloproteases in cartilage biology. Dis Markers 2016:4895050
Sahni M, Raz R, Coffin JD, Levy D, Basilico C (2001) STAT1 mediates the increased apoptosis and reduced chondrocyte proliferation in mice overexpressing FGF2. Development 128:2119–2129
Millward-Sadler SJ, Khan NS, Bracher MG, Wright MO, Salter DM (2006) Roles for the interleukin-4 receptor and associated JAK/STAT proteins in human articular chondrocyte mechanotransduction. Osteoarthr Cartilage 14:991–1001
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Number 81702136).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were performed in accordance with the guidelines of the Institutional Animal Ethics Committee of Changchun University of Chinese Medicine (No. ccucm-2017-0015).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, Z., Yao, B. & Zhao, D. Runx3 regulates chondrocyte phenotype by controlling multiple genes involved in chondrocyte proliferation and differentiation. Mol Biol Rep 47, 5773–5792 (2020). https://doi.org/10.1007/s11033-020-05646-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05646-6