Abstract
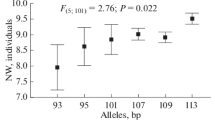
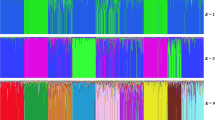
The emu (Dromaius novaehollandiae) is a useful poultry animal farmed for fat, meat, and eggs. Genetic structure and relationships among farmed emu populations in Japan are unknown and the number of microsatellite markers for genetic analysis of the emu is insufficient. In this study, we isolated 16 microsatellites from the emu genome and developed ten new microsatellite markers. These microsatellite markers were used to characterize three farm emu populations in Japan. The number of alleles ranged from 3 to 13 and the expected (HE) and observed heterozygosity (HO) of these microsatellite loci was 0.187–0.802 and 0.179–0.647, respectively. The polymorphic information content ranged from 0.176 to 0.786. Positive inbreeding coefficient (FIS) values were detected in all tested populations, and they ranged from 0.027 to 0.540. These results suggest that farm populations of the emu in Japan resulted from inbreeding. The fixation index (FST) values ranged from 0.026 to 0.061, and phylogenetic trees and population structure analysis confirmed no definitive genetic differentiation among the three populations. Therefore, these populations are at a relatively low level of genetic differentiation at present. The microsatellite markers developed in our study can be utilized for genetic analysis and preservation of genetic resources in the emu.

Similar content being viewed by others
References
Menon DG, Bennett DC, Schaefer AM, Cheng KM (2013) Hematological and serum biochemical profile of farm emus (Dromaius novaehollandiae) at the onset of their breeding season. Poult Sci 92:935–944
Yokohama M, Jinushi H, Imai S, Ikeya K (2014) Effects of pairing on egg laying in the emu. J Agric Sci Tokyo Univ Agric 58:229–234
Snowden JM, Whitehouse MW (1997) Anti-inflammatory activity of emu oils in rats. Inflammopharmacology 5:127–132
López A, Sims DE, Ablett RF, Skinner RE, Léger LW, Lariviere CM, Jamieson LA, Martínez-Burnes J, Zawadzka GG (1999) Effect of emu oil on auricular inflammation induced with croton oil in mice. Am J Vet Res 60:1558–1561
Yoganathan S, Nicolosi R, Wilson T, Handelman G, Scollin P, Tao R, Binford P, Orthoefer F (2003) Antagonism of croton oil inflammation by topical emu oil in CD-1 mice. Lipids 38:603–607
Lindsay RJ, Geier MS, Yazbeck R, Butler RN, Howarth GS (2010) Orally administered emu oil decreases acute inflammation and alters selected small intestinal parameters in a rat model of mucositis. Br J Nutr 104:513–519
Abimosleh SM, Lindsay RJ, Butler RN, Cummins AG, Howarth GS (2012) Emu oil increases colonic crypt depth in a rat model of ulcerative colitis. Dig Dis Sci 57:887–896
Chartier LC, Howarth GS, Lawrance IC, Trinder D, Barker SJ, Mashtoub S (2018) Emu oil improves clinical indicators of disease in a mouse model of colitis-associated colorectal cancer. Dig Dis Sci 63:135–145
Miyashita T, Minami K, Ito M, Koizumi R, Sagane Y, Watanabe T, Niwa K (2018) Emu oil reduces LPS-induced production of nitric oxide and TNF-α but not phagocytosis in RAW 264 macrophages. J Oleo Sci 67:471–477
Ito M, Minami K, Sagane Y, Watanabe T, Niwa K (2016) Data on melanin production in B16F1 melanoma cells in the presence of emu oil. Data Brief 9:1056–1059
Zanardo V, Giarrizzo D, Volpe F, Giliberti L, Straface G (2017) Emu oil-based lotion effects on neonatal skin barrier during transition from intrauterine to extrauterine life. Clin Cosmet Investig Dermatol 10:299–303
Taylor EL, Vercoe P, Cockrem J, Groth D, Wetherall JD, Martin GB (1999) Isolation and characterization of microsatellite loci in the emu, Dromaius novaehollandiae, and cross-species amplification within Ratitae. Mol Ecol 8:1963–1964
Yáñez JM, González R, Angulo J, Vidal R, Santos JL, Martínez V (2008) Characterization of new microsatellite markers derived from sequence databases for the emu (Dromaius novaehollandiae). Mol Ecol Resour 8:1442–1444
Koshiishi Y, Okubo MM, Shimoi G, Hirayama H, Souma K, Wada K (2019) Genetic diversity of emu population in a Japanese farm based on microsatellite DNA analysis. J Agric Sci Tokyo Univ Agric 64:42–48
De Kloet SR (2001) Development of a CAPS (cleaved amplified polymorphics sequence) assay for sex identification of the emu (Dromaius novaehollandiae). Mol Ecol Notes 1:273–275
Koshiishi Y, Wada K (2018) A simplified protocol for molecular sexing in the emu (Dromaius novaehollandiae). Poult Sci 97:1117–1119
Glenn TC, Schable NA (2005) Isolating microsatellite DNA loci. Methods Enzymol 395:202–222
Seki Y, Yokohama M, Ishikawa D, Ikehara N, Wada K, Nomura K, Amano T, Kikkawa Y (2012) Development and characterization of 260 microsatellite loci in the domestic goat, Capra hircus. Anim Genet 43:365–366
Tada T, Seki Y, Kameyama Y, Kikkawa Y, Wada K (2016) Characterization and application of newly developed polymorphic microsatellite markers in the Ezo red fox (Vulpes vulpes schrencki). Genet Mol Res. https://doi.org/10.4238/gmr15049104
Kalinowski ST, Taper ML, Marshall TC (2007) Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 16:1099–1106
Rousset F (2008) genepop'007: a complete re-implementation of the genepop software for Windows and Linux. Mol Ecol Resour 8:103–106
Chakraborty R, Jin L (1993) A unified approach to study hypervariable polymorphisms: statistical considerations of determining relatedness and population distances. EXS 67:153–175
Nei M, Tajima F, Tateno Y (1983) Accuracy of estimated phylogenetic trees from molecular data. II. Gene frequency data. J Mol Evol 19:153–170
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Felsenstein J (1989) PHYLIP—phylogeny inference package (version 3.2). Cladistics 5:164–166
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Falush D, Stephens M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164:1567–1587
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Campana MG, Hunt HV, Jones H, White J (2011) CorrSieve: software for summarizing and evaluating Structure output. Mol Ecol Resour 11:349–352
Le Duc D, Renaud G, Krishnan A, Almén MS, Huynen L, Prohaska SJ, Ongyerth M, Bitarello BD, Schiöth HB, Hofreiter M, Stadler PF, Prüfer K, Lambert D, Kelso J, Schöneberg T (2015) Kiwi genome provides insights into evolution of a nocturnal lifestyle. Genome Biol 16:147
Hammond EL, Lymbery AJ, Martin GB, Groth D, Wetherall JD (2002) Microsatellite analysis of genetic diversity in wild and farmed emus (Dromaius novaehollandiae). J Hered 93:376–380
Kawka M, Parada R, Jaszczak K, Horbańczuk JO (2012) The use of microsatellite polymorphism in genetic mapping of the ostrich (Struthio camelus). Mol Biol Rep 39:3369–3374
Yokohama M (2016) Emu for bioindustry. J Agric Sci Tokyo Univ Agric 60:189–204 (In Japanese with English abstract)
Acknowledgements
We greatly thank Okhotsk Emu Farm (Abashiri, Hokkaido, Japan), Tohoku Safari Park (Nihonmatu, Fukushima, Japan), and Japan Eco System, Co., Ltd (Chikusino, Fukuoka, Japan) for assistance with this study. We also thank many students from Tokyo University of Agriculture for their help with sample collection.
Funding
This work was supported by the Grant-in-Aid for Focused Study Project for Graduate School and the Grant-in-Aid for University Strategic Study Project from Tokyo University of Agriculture.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures involving animals met the guidelines described in “The Proper Conduct of Animal Experiments,” proposed by the Science Countil of Japan, and were approved by the Ethical Care and Use of Animals Committee at the Tokyo University of Agriculture (Approval Number: 270049). The authors hereby declare that (a) the present manuscript has not been submitted to any other journal simultaneously; (b) the present manuscript has not been published previously; (c) the present manuscript covers a completed study (i.e., microsatellite marker development in the emu Dromaius novaehollandiae); (d) the authors have not fabricated nor manipulated any data presented in the manuscript; (e) no data, text or theories by others are presented by the authors as their own. All references to other authors´ works are duly marked in the text. No humans were subject to the present study.
Informed consent
All authors consent to the submission of this manuscript to the journal Molecular Biology Reports.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
The prediction of the most likely number of ancestors in Japanese farmed emu populations. The line graph (the right Y axis colored by red) and bars (the left Y axis colored by blue) indicate ΔK values and L(K) values when K = 2–7. (TIF 977 kb)
Rights and permissions
About this article
Cite this article
Koshiishi, Y., Murata-Okubo, M., Fujisawa, Si. et al. Development and characterization of ten novel microsatellite loci for the emu (Dromaius novaehollandiae) and genetic diversity of Japanese farm populations. Mol Biol Rep 47, 2521–2527 (2020). https://doi.org/10.1007/s11033-020-05335-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05335-4