Abstract
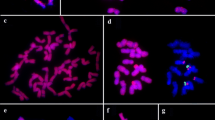
The genus Urochloa P. Beauv. [syn. Brachiaria (Trin.) Griseb.] comprises species of great economic relevance as forages. The genomic constitution for the allotetraploid species Urochloa brizantha (cv. Marandu) and Urochloa decumbens (cv. Basilisk) and the diploid Urochloa ruziziensis was previously proposed as BBB1B1, B1B1B2B2 and B2B2, respectively. Evidence indicates U. ruziziensis as the ancestral donor of genome B2 in U. decumbens allotetraploidy, but the origin of the genomes B and B1 is still unknown. There are diploid genotypes of U. brizantha and U. decumbens that may be potential ancestors of the tetraploids. The aim of this study was to determine the genomic constitution and relationships between genotypes of U. brizantha (2x and 4x), U. decumbens (2x and 4x) and U. ruziziensis (2x) via genomic in situ hybridization (GISH). Additionally, chromosome number and genome size were verified for the diploid genotypes. The diploids U. brizantha and U. decumbens presented 2n = 2x = 18 chromosomes and DNA content of 1.79 and 1.44 pg, respectively. The GISH analysis revealed high homology between the diploids U. brizantha and U. decumbens, which suggests relatively short divergence time. The GISH using genomic probes from the diploid accessions on the tetraploid accessions’ chromosomes presented similar patterns, highlighting the genome B1 present in both of the tetraploids. Based on GISH results, the genomic constitution was proposed for the diploid genotypes of U. brizantha (B1B1) and U. decumbens (B1′B1′) and both were pointed as donors of genome B1 (or B1′), present in the allotetraploid genotypes.




Similar content being viewed by others
Abbreviations
- GISH:
-
Genomic in situ hybridization
- RAPD:
-
Random amplification of polymorphic DNA
- CTAB:
-
Cetyltrimethylammonium bromide
- gDNA:
-
Genomic DNA
- SSC:
-
Saline sodium citrate
- TNT:
-
Tris-NaCl-Tween-20
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- H3K4me2:
-
Dimethylation of the lysine residue at 4th position on the N-terminal tail of histone 3
- H3K9me2:
-
Dimethylation of the lysine residue at 9th position on the N-terminal tail of histone 3
- FISH:
-
Fluorescent in situ hybridization
- rDNA:
-
Ribosomal DNA
References
Stevens PF (2001 onwards) Angiosperm Phylogeny Website. Version 14, July 2018 [and more or less continuously updated since]. http://www.mobot.org/MOBOT/research/APweb/. Accessed 2018
Jank L, Barrios SC, Valle CB et al (2014) The value of improved pastures to Brazilian beef production. Crop Pasture Sci 65:1132–1137
Valle CB, Jank L, Resende RM (2009) O melhoramento de forrageiras tropicais no Brasil. Rev Ceres 56:460–472
Valle CB, Savidan YH (1996) Genetics cytogenetics and reproductive biology of Brachiaria. In: Miles JW, Maass BL, Valle CB (eds) Brachiaria biology agronomy and improvement. Empresa Brasilera de Pesquisa Agropecuaria, Brasilia, pp 163–180
Assis GML, Euclydes RF, Cruz CD, Valle CB (2002) Genetic divergence in Brachiaria species. Crop Breed Appl Biotechnol 2:331–338. https://doi.org/10.12702/1984-7033.v02n03a02
Renvoize SA, Clayton WB, Kabuye CHS (1996) Morphology, taxonomy and natural distribution of Brachiaria (Trin.) Griseb. In: Kuumble V, Miles JW, Maass BL (eds) Brachiaria: biology, agronomy and improvement. Empresa Brasilera de Pesquisa Agropecuaria, Brasilia, pp 1–15
Ambiel AC, Guaberto LM, Vanderlei TM, Neto NBM (2008) Agrupamento de acessos e cultivares de três espécies de brachiaria por RAPD. Acta Sci Agron 30:457–464. https://doi.org/10.4025/actasciagron.v30i4.5298
Ambiel AC, Neto NBM, Guaberto LM, Vanderlei TM (2010) Brachiaria germplasm dissimilarity as shown by RAPD markers. Crop Breed Appl Biotechnol 10:55–64
Pessoa-Filho M, Martins AM, Ferreira ME (2017) Molecular dating of phylogenetic divergence between Urochloa species based on complete chloroplast genomes. BMC Genomics 18:516. https://doi.org/10.1186/s12864-017-3904-2
Triviño NJ, Perez JG, Recio ME et al (2017) Genetic diversity and population structure of Brachiaria species and breeding populations. Crop Sci 57:2633–2644. https://doi.org/10.2135/cropsci2017.01.0045
Basappa GP, Muniyamma M, Chinnappa CC (1987) An investigation of chromosome numbers in the genus Brachiaria (Poaceae: Paniceae) in relation to morphology and taxonomy. Can J Bot 65:2297–2309
Valle CB, Pagliarini MS (2009), Cytogenetics, and breeding of Brachiaria. In: Singh RJ (ed) Genetic resources, chromosome engineering, and crop improvement. CRC Press, Boca Raton, pp 103–143
Mendes-Bonato AB, Filho RGJ, Pagliarini MS et al (2002) Unusual cytological patterns of microsporogenesis in Brachiaria decumbens: abnormalities in spindle and defective cytokinesis causing precocious cellularization. Cell Biol Int 26:641–646. https://doi.org/10.1006/cbir.2002.0929
Mendes-Bonato AB, Pagliarini MS, Forli F et al (2002) Chromosome numbers and microsporogenesis in Brachiaria brizantha (Gramineae). Euphytica 125:419–425
Mendes-Bonato AB, Pagliarini MS, Silva N, Valle CB (2001) Meiotic instability in invader plants of signal grass Brachiaria decumbens Stapf (Gramineae). Acta Sci 23:619–625
Mendes-Bonato AB, Risso-Pascotto C, Pagliarini MS, Valle CB (2006) Cytogenetic evidence for genome elimination during microsporogenesis in interspecific hybrid between Brachiaria ruziziensis and B. brizantha (Poaceae). Genet Mol Biol 29:711–714
Mendes DV, Boldrini KR, Mendes-Bonato AB et al (2006) Cytological evidence of natural hybridization in Brachiaria brizantha Stapf (Gramineae). Bot J Linn Soc 150:441–446
Paula CMP, Souza Sobrinho F, Techio VH (2017) Genomic constitution and relationship in Urochloa (Poaceae) species and hybrids. Crop Sci 57:2605–2616. https://doi.org/10.2135/cropsci2017.05.0307
Lutts S, Ndikumana J, Louant BP (1991) Fertility of Brachiaria ruziziensis in interspecific crosses with Brachiaria decumbens and Brachiaria brizantha: meiotic behavior, pollen viability and seed set. Euphytica 57:267–274
Ndikumana J (1985) Etude de l’hybridation entre espèces apomitcques et sexuées dans le genre Brachiaria. Universite Catholique de Louvain, Belgium
Dong F, McGrath JM, Helgeson JP, Jiang J (2001) The genetic identity of alien chromosomes in potato breeding lines revealed by sequential GISH and FISH analyses using chromosome-specific cytogenetic DNA markers. Genome. https://doi.org/10.1139/gen-44-4-729
Doyle J, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus (Madison) 12:39–40
Altinordu F, Pesuzzi L, Yu Y, He X (2016) A tool for the analysis of chromosomes: karyotype. Taxon 65(3):586–592
Heslop-Harrison JSP, Schwarzacher T (2011) Organisation of the plant genome in chromosomes. Plant J 66:18–33. https://doi.org/10.1111/j.1365-313X.2011.04544.x
Dolezel J (1997) Application of flow cytometry for the study of plant genomes. J Appl Genet 3:285–302
Nielen S, Almeida LM, Carneiro VTC, Araujo ACG (2009) Physical mapping of rDNA genes corroborates allopolyploid origin in apomictic Brachiaria brizantha. Sex Plant Reprod 23:45–51. https://doi.org/10.1007/s00497-009-0124-1
De Penteado MI, Santos ACM, Rodrigues IF et al (2000) Determinação de poliploidia e avaliação da quantidade de DNA total em diferentes espécies de gênero Brachiaria. Bol Pesqui Embrapa 11:1–32
Pinheiro AA (2000) Duplication of the chromosome number of diploid Brachiaria brizantha plants using colchicine. Plant Cell Rep 18:274–278
Ricci GCL, Souza-Kaneshima AM, Felismino MF et al (2011) Chromosome numbers and meiotic analysis in the pre-breeding of Brachiaria decumbens (Poaceae). Indian Acad Sci 90:289–294
Ishigaki G, Gondo T, Ebina M et al (2010) Estimation of genome size in Brachiaria species. Grassl Sci 56:240–242. https://doi.org/10.1111/j.1744-697X.2010.00200.x
Timbó AL, Pereira RC, Souza Sobrinho F, Davide LC (2014) Nuclear DNA content and chromosome numbereira in Brachiaria spp. genotypes. Rev Cienc Agron 45:62–67
Leitch IJ, Soltis DE, Soltis PS, Bennett MD (2005) Evolution of DNA amounts across land plants (Embryophyta). Ann Bot 95:207–217. https://doi.org/10.1093/aob/mci014
Bennetzen JL, Ma J, Devos KM (2005) Mechanisms of recent genome size variation in flowering plants. Ann Bot 95:127–132. https://doi.org/10.1093/aob/mci008
Belyayev A, Raskina O, Nevo E (2001) Evolutionary dynamics and chromosomal distribution of repetitive sequences on chromosomes of Aegilops speltoides revealed by genomic in situ hybridization. Heredity (Edinburgh) 86:738–742. https://doi.org/10.1046/j.1365-2540.2001.00891.x
Maluszynska J, Hasterok R (2005) Identification of individual chromosomes and parental genomes in Brassica juncea using GISH and FISH. Cytogenet Genome Res 109:310–314. https://doi.org/10.1159/000082414
Paula CM, Sobrinho FS, Techio VH (2016) Chromosomal distribution of H3K4me2, H3K9me2 and 5-methylcytosine: variations associated with polyploidy and hybridization in Brachiaria (Poaceae). Plant Cell Rep 35:1359–1369
Li C, Zhang D, Ge S et al (2001) Identification of genome constitution of Oryza malampuzhaensis, O. minuta, and O. punctata by multicolour genomic in situ hybridization. Theor Appl Genet 103:204–211
Jenkins G, Mur L, Bablak P et al (2004) Prospects for functional genomics in a new model grass. In: Leister D (ed) Plant functional genomics. CRC Press, Boca Raton
Roa F, Guerra M (2015) Non-random distribution of 5S rDNA sites and its association with 45S rDNA in plant chromosomes. Cytogenet Genome Res 146:243–249. https://doi.org/10.1159/000440930
Watson JM, Riha K (2010) Comparative biology of telomeres: where plants stand. FEBS Lett 584:3752–3759. https://doi.org/10.1016/j.febslet.2010.06.017
Majka J, Majka M, Kwiatek M, Wiśniewska H (2017) Similarities and differences in the nuclear genome organization within Pooideae species revealed by comparative genomic in situ hybridization (GISH). J Appl Genet 58:151–161. https://doi.org/10.1007/s13353-016-0369-y
Salina EA, Sergeeva EM, Adonina IG et al (2009) Isolation and sequence analysis of the wheat B genome subtelomeric DNA. BMC Genomics 10:414. https://doi.org/10.1186/1471-2164-10-414
Nani TF, Pereira DL, Souza Sobrinho F, Techio VH (2016) Physical map of repetitive DNA sites in Brachiaria spp.: intravarietal and interspecific polymorphisms. Crop Sci 56:1769–1783. https://doi.org/10.2135/cropsci2015.12.0760
Flavell RB, Gale MD, O’dell M et al (1993) Molecular organization of genes and repeats in the large cereal genomes and implications for the isolation of genes by chromosome walking. Chromosomes today. Springer, Dordrecht, pp 199–213
Schnable PS, Ware D, Fulton RS et al (2009) The B73 maize genome: complexity, diversity, and dynamics. Science 326:1112–1115. https://doi.org/10.1126/science.1178534
Biscotti MA, Olmo E, Heslop-Harrison JS (2015) Repetitive DNA in eukaryotic genomes. Chromosom Res 23:415–420. https://doi.org/10.1007/s10577-015-9499-z
López-Flores I, Garrido-Ramos MA (2012) The repetitive DNA content of eukaryotic genomes. Repetitive DNA 7:1–28. https://doi.org/10.1159/000337118
Morrone O, Zuloaga FO (1992) Revision de las especies sudamericanas nativas e introducidas de los generos Brachiaria y Urochloa (Poaceae: Panicoideae: Paniceae). Darwiniana 31:43–109
Maass BL (2004) Identifying and naming Brachiaria species. In: Miles JW, Valle CB (eds) Brachiaria: biology, agronomy and improvement. Empresa Brasilera de Pesquisa Agropecuaria, Brasilia, pp 9–12
Akiyama Y, Yamada-Akiyama H, Ebina M (2010) Morphological diversity of chromosomes bearing ribosomal DNA loci in Brachiaria species. Grassl Sci 56:217–223. https://doi.org/10.1111/j.1744-697X.2010.00197.x
Nielen S, Almeida LM, Carneiro VTC, Araujo ACG (2010) Physical mapping of rDNA genes corroborates allopolyploid origin in apomictic Brachiaria brizantha. Sex Plant Reprod 23:45–51. https://doi.org/10.1007/s00497-009-0124-1
Renny-Byfield S, Wendel JF (2014) Doubling down on genomes: polyploidy and crop plants. Am J Bot 101:1711–1725. https://doi.org/10.3732/ajb.1400119
Gaut B, Thierry d’Ennenquin M, Peek A, Sawkins N (2000) Maize as a model for the evolution of plant nuclear genomes. Proc Natl Acad Sci U S A 97:7008–7015
Ermolaeva M, Wu M, Eisen J, Salzberg S (2003) The age of the Arabidopsis thaliana genome duplication. Plant Mol Biol 51:859–866
Doyle JA, Endress PK (2000) Morphological phylogenetic analysis of basal angiosperms: comparison and combination with molecular data. Int J Plant Sci 161:121–153
Acknowledgements
The authors thank the support of the Foundation for Research Support of the State of Minas Gerais (FAPEMIG), the Coordination for the Improvement of Higher Education Personnel (CAPES), the National Council for Scientific and Technological Development (CNPq) for financial support for the development of this study. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Contributions
CTRC—carried oed out flow cytometry analysis, helped prepared slides and image processing. SCLB and CBV—responsible for the breeding program of Urochloa; provided hybrid seeds of Urochloa and reviewed the manuscript. GAT—helped the GISH analysis and has been involved in drafting the manuscript. VHT—conceived the study, participated in its design and coordination and helped to draft the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This study is part of C.T.R. Correa’s thesis and the abstract can be found in the repository: http://repositorio.ufla.br/jspui/handle/1/33642.
Rights and permissions
About this article
Cite this article
Corrêa, C.T.R., Bonetti, N.G.Z., Barrios, S.C.L. et al. GISH-based comparative genomic analysis in Urochloa P. Beauv.. Mol Biol Rep 47, 887–896 (2020). https://doi.org/10.1007/s11033-019-05179-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05179-7